Identification of LMAN1- and SURF4-Dependent Secretory Cargoes
- PMID: 37844105
- PMCID: PMC10629478
- DOI: 10.1021/acs.jproteome.3c00259
Identification of LMAN1- and SURF4-Dependent Secretory Cargoes
Abstract
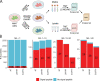
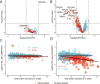
Most proteins secreted into the extracellular space are first recruited from the endoplasmic reticulum into coat protein complex II (COPII)-coated vesicles or tubules that facilitate their transport to the Golgi apparatus. Although several secreted proteins have been shown to be actively recruited into COPII vesicles and tubules by the cargo receptors LMAN1 and SURF4, the full cargo repertoire of these receptors is unknown. We now report mass spectrometry analysis of conditioned media and cell lysates from HuH7 cells CRISPR targeted to inactivate the LMAN1 or SURF4 gene. We found that LMAN1 has limited clients in HuH7 cells, whereas SURF4 traffics a broad range of cargoes. Analysis of putative SURF4 cargoes suggests that cargo recognition is governed by complex mechanisms rather than interaction with a universal binding motif..
Keywords: COPII trafficking; cargo receptor; secretome.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Update of
-
Identification of LMAN1 and SURF4 dependent secretory cargoes.bioRxiv [Preprint]. 2023 Apr 6:2023.04.06.535922. doi: 10.1101/2023.04.06.535922. bioRxiv. 2023. Update in: J Proteome Res. 2023 Nov 3;22(11):3439-3446. doi: 10.1021/acs.jproteome.3c00259. PMID: 37066360 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

