Co-option of a conserved host glutamine transporter facilitates aphid/ Buchnera metabolic integration
- PMID: 37844224
- PMCID: PMC10614625
- DOI: 10.1073/pnas.2308448120
Co-option of a conserved host glutamine transporter facilitates aphid/ Buchnera metabolic integration
Abstract
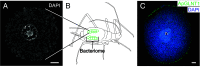
Organisms across the tree of life colonize novel environments by partnering with bacterial symbionts. These symbioses are characterized by intimate integration of host/endosymbiont biology at multiple levels, including metabolically. Metabolic integration is particularly important for sap-feeding insects and their symbionts, which supplement nutritionally unbalanced host diets. Many studies reveal parallel evolution of host/endosymbiont metabolic complementarity in amino acid biosynthesis, raising questions about how amino acid metabolism is regulated, how regulatory mechanisms evolve, and the extent to which similar mechanisms evolve in different systems. In the aphid/Buchnera symbiosis, the transporter ApGLNT1 (Acyrthosiphon pisum glutamine transporter 1) supplies glutamine, an amino donor in transamination reactions, to bacteriocytes (where Buchnera reside) and is competitively inhibited by Buchnera-supplied arginine-consistent with a role regulating amino acid metabolism given host demand for Buchnera-produced amino acids. We examined how ApGLNT1 evolved a regulatory role by functionally characterizing orthologs in insects with and without endosymbionts. ApGLNT1 orthologs are functionally similar, and orthology searches coupled with homology modeling revealed that GLNT1 is ancient and structurally conserved across insects. Our results indicate that the ApGLNT1 symbiotic regulatory role is derived from its ancestral role and, in aphids, is likely facilitated by loss of arginine biosynthesis through the urea cycle. Given consistent loss of host arginine biosynthesis and retention of endosymbiont arginine supply, we hypothesize that GLNT1 is a general mechanism regulating amino acid metabolism in sap-feeding insects. This work fills a gap, highlighting the broad importance of co-option of ancestral proteins to novel contexts in the evolution of host/symbiont systems.
Keywords: A. pisum; Buchnera; amino acid metabolism; gene co-option; symbiosis.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
-
- Hackett J., Migration of the plastid genome to the nucleus in a peridinin dinoflagellate. Curr. Biol. 14, 213–218 (2004). - PubMed
-
- Husnik F., et al. , Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 153, 1567–1578 (2013). - PubMed
-
- Nakabachi A., Ishida K., Hongoh Y., Ohkuma M., Miyagishima S., Aphid gene of bacterial origin encodes a protein transported to an obligate endosymbiont. Curr. Biol. 24, R640–R641 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

