Fibrin hydrogels fortified with FGF-7/10 and laminin-1 peptides promote regeneration of irradiated salivary glands
- PMID: 37844750
- PMCID: PMC10908308
- DOI: 10.1016/j.actbio.2023.10.013
Fibrin hydrogels fortified with FGF-7/10 and laminin-1 peptides promote regeneration of irradiated salivary glands
Abstract
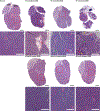
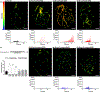
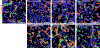
Ionizing radiation, commonly used for head and neck cancer treatment, typically damages the salivary glands, resulting in hyposalivation. The development of treatments to restore this lost function is crucial for improving the quality of life for patients suffering from this condition. To address this clinical need, we have developed an innovative hydrogel by chemically conjugating laminin-1 peptides (A99 and YIGSR) and growth factors, FGF-7 and FGF-10, to fibrin hydrogels. Our results demonstrate that FGF-7/10 and laminin-1 peptides fortified fibrin hydrogel [enhanced laminin-1 peptides fibrin hydrogel (Ep-FH)] promotes salivary gland regeneration and functionality by improving epithelial tissue organization, establishing a healthy network of blood vessels and nerves, while reducing fibrosis in a head and neck irradiated mouse model. These results indicate that fibrin hydrogel-based implantable scaffolds containing pro-regenerative signals promote sustained secretory function of irradiated salivary glands, offering a potential alternative treatment for hyposalivation in head and neck cancer patients undergoing radiation treatment. These unique findings emphasize the potential of fibrin hydrogel-based implantable scaffolds enriched with pro-regenerative signals in sustaining the secretory function of irradiated salivary glands and offer a promising alternative treatment for addressing hyposalivation in head and neck cancer patients undergoing radiation therapy. STATEMENT OF SIGNIFICANCE: Radiation therapies used to treat head and neck cancers often result in damaged salivary gland, leading to severe dryness of the oral cavity. In this study, we engineered FGF-7 and FGF-10 and immobilized them into L1p-FH. The resulting hydrogel, Ep-FH, restored irradiated salivary gland functionality by enhancing epithelial tissue organization, promoting the development of a healthy network of blood vessels and nerves as well as reduction of fibrosis.
Keywords: Fibrin hydrogels; Growth factor; Saliva; Submandibular glands; Tissue engineering.
Copyright © 2023 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Carlson ER, Schlieve T, Salivary gland malignancies, Oral Maxillofac. Surg. Clin. North Am. 31 (1) (2019) 125–144. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics, 2022, CA Cancer J. Clin. 72 (1) (2022) 7–33. - PubMed
-
- Adkins D, Ley J, Oppelt P, Gay HA, Daly M, Paniello RC, Jackson R, Pipkorn P, Rich J, Zevallos J, Trinkaus K, Thorstad W, Impact on health-related quality of life of induction chemotherapy compared with concurrent cisplatin and radiation therapy in patients with head and neck cancer, Clin. Oncol. 31 (9) (2019) e123–e131. - PubMed
-
- Braga MA, Tarzia O, Bergamaschi CC, Santos FA, Andrade ED, Groppo FC, Comparison of the effects of pilocarpine and cevimeline on salivary flow, Int. J. Dent. Hyg. 7 (2) (2009) 126–130. - PubMed
-
- Turner MD, Hyposalivation and xerostomia: etiology, complications, and medical management, Dent. Clin. North Am. 60 (2) (2016) 435–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

