Protein 4.1N Plays a Cell Type-Specific Role in Hippocampal Glutamatergic Synapse Regulation
- PMID: 37845032
- PMCID: PMC10711697
- DOI: 10.1523/JNEUROSCI.0185-23.2023
Protein 4.1N Plays a Cell Type-Specific Role in Hippocampal Glutamatergic Synapse Regulation
Abstract
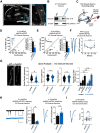
Many glutamatergic synapse proteins contain a 4.1N protein binding domain. However, a role for 4.1N in the regulation of glutamatergic neurotransmission has been controversial. Here, we observe significantly higher expression of protein 4.1N in granule neurons of the dentate gyrus (DG granule neurons) compared with other hippocampal regions. We discover that reducing 4.1N expression in rat DG granule neurons of either sex results in a significant reduction in glutamatergic synapse function that is caused by a decrease in the number of glutamatergic synapses. By contrast, we find reduction of 4.1N expression in hippocampal CA1 pyramidal neurons has no impact on basal glutamatergic neurotransmission. We also find 4.1N's C-terminal domain (CTD) to be nonessential to its role in the regulation of glutamatergic synapses of DG granule neurons. Instead, we show that 4.1N's four-point-one, ezrin, radixin, and moesin (FERM) domain is essential for supporting synaptic AMPA receptor (AMPAR) function in these neurons. Altogether, this work demonstrates a novel, cell type-specific role for protein 4.1N in governing glutamatergic synapse function.SIGNIFICANCE STATEMENT Glutamatergic synapses exhibit immense molecular diversity. In comparison to heavily studied Schaffer collateral, CA1 glutamatergic synapses, significantly less is known about perforant path-dentate gyrus (DG) synapses. Our data demonstrate that compromising 4.1N function in CA1 pyramidal neurons produces no alteration in basal glutamatergic synaptic transmission. However, in DG granule neurons, compromising 4.1N function leads to a significant decrease in the strength of glutamatergic neurotransmission at perforant pathway synapses. Together, our data identifies 4.1N as a cell type-specific regulator of synaptic transmission within the hippocampus and reveals a unique molecular program that governs perforant pathway synapse function.
Keywords: 4.1N; AMPA receptor; dendritic spines; dentate gyrus; synapse.
Copyright © 2023 the authors.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
