Different Faces of Medial Beta-Band Activity Reflect Distinct Visuomotor Feedback Signals
- PMID: 37845035
- PMCID: PMC10711699
- DOI: 10.1523/JNEUROSCI.2238-22.2023
Different Faces of Medial Beta-Band Activity Reflect Distinct Visuomotor Feedback Signals
Abstract
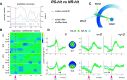
Beta-band (13-35 Hz) modulations following reward, task outcome feedback, and error have been described in cognitive and/or motor adaptation tasks. Observations from different studies are, however, difficult to conciliate. Among the studies that used cognitive response selection tasks, several reported an increase in beta-band activity following reward, whereas others observed increased beta power after negative feedback. Moreover, in motor adaptation tasks, an attenuation of the postmovement beta rebound follows a movement execution error induced by visual or mechanical perturbations. Given that kinematic error typically leads to negative task-outcome feedback (e.g., target missed), one may wonder how contradictory modulations, beta power decrease with movement error versus beta power increase with negative feedback, may coexist. We designed a motor adaptation task in which female and male participants experience varied feedbacks-binary success/failure feedback, kinematic error, and sensory-prediction error-and demonstrate that beta-band modulations in opposite directions coexist at different spatial locations, time windows, and frequency ranges. First, high beta power in the medial frontal cortex showed opposite modulations well separated in time when compared in success and failure trials; that is, power was higher in success trials just after the binary success feedback, whereas it was lower in the postmovement period compared with failure trials. Second, although medial frontal high-beta activity was sensitive to task outcome, low-beta power in the medial parietal cortex was strongly attenuated following movement execution error but was not affected by either the outcome of the task or sensory-prediction error. These findings suggest that medial beta activity in different spatio-temporal-spectral configurations play a multifaceted role in encoding qualitatively distinct feedback signals.SIGNIFICANCE STATEMENT Beta-band activity reflects neural processes well beyond sensorimotor functions, including cognition and motivation. By disentangling alternative spatio-temporal-spectral patterns of possible beta-oscillatory activity, we reconcile a seemingly discrepant literature. First, high-beta power in the medial frontal cortex showed opposite modulations separated in time in success and failure trials; power was higher in success trials just after success feedback and lower in the postmovement period compared with failure trials. Second, although medial frontal high-beta activity was sensitive to task outcome, low-beta power in the medial parietal cortex was strongly attenuated following movement execution error but was not affected by the task outcome or the sensory-prediction error. We propose that medial beta activity reflects distinct feedback signals depending on its anatomic location, time window, and frequency range.
Keywords: EEG; beta-band oscillations; feedback processing; motor learning; reaching; sensory-prediction error.
Copyright © 2023 the authors.
Figures





References
-
- Alayrangues J, Torrecillos F, Jahani A, Malfait N (2019) Error-related modulations of the sensorimotor post-movement and foreperiod beta-band activities arise from distinct neural substrates and do not reflect efferent signal processing. Neuroimage 184:10–24. 10.1016/j.neuroimage.2018.09.013 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
