Distinct ACC Neural Mechanisms Underlie Authentic and Transmitted Anxiety Induced by Maternal Separation in Mice
- PMID: 37845036
- PMCID: PMC10697407
- DOI: 10.1523/JNEUROSCI.0558-23.2023
Distinct ACC Neural Mechanisms Underlie Authentic and Transmitted Anxiety Induced by Maternal Separation in Mice
Abstract
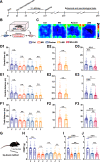
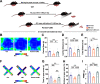
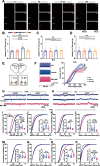
It is known that humans and rodents are capable of transmitting stress to their naive partners via social interaction. However, a comprehensive understanding of transmitted stress, which may differ from authentic stress, thus revealing unique neural mechanisms of social interaction resulting from transmitted stress and the associated anxiety, is missing. We used, in the present study, maternal separation (MS) as a stress model to investigate whether MS causes abnormal behavior in adolescence. A key concern in the analysis of stress transmission is whether the littermates of MS mice who only witness MS stress ("Partners") exhibit behavioral abnormalities similar to those of MS mice themselves. Of special interest is the establishment of the neural mechanisms underlying transmitted stress and authentic stress. The results show that Partners, similar to MS mice, exhibit anxiety-like behavior and hyperalgesia after witnessing littermates being subjected to early-life repetitive MS. Electrophysiological analysis revealed that mice subjected to MS demonstrate a reduction in both the excitatory and inhibitory synaptic activities of parvalbumin interneurons (PVINs) in the anterior cingulate cortex (ACC). However, Partners differed from MS mice in showing an increase in the number and excitability of GABAergic PVINs in the ACC and in the ability of chemogenetic PVIN inactivation to eliminate abnormal behavior. Furthermore, the social transfer of anxiety-like behavior required intact olfactory, but not visual, perception. This study suggests a functional involvement of ACC PVINs in mediating the distinct neural basis of transmitted anxiety.SIGNIFICANCE STATEMENT The anterior cingulate cortex (ACC) is a critical brain area in physical and social pain and contributes to the exhibition of abnormal behavior. ACC glutamatergic neurons have been shown to encode transmitted stress, but it remains unclear whether inhibitory ACC neurons also play a role. We evaluate, in this study, ACC neuronal, synaptic and network activities and uncover a critical role of parvalbumin interneurons (PVINs) in the expression of transmitted stress in adolescent mice who had witnessed MS of littermates in infancy. Furthermore, inactivation of ACC PVINs blocks transmitted stress. The results suggest that emotional contagion has a severe effect on brain function, and identify a potential target for the treatment of transmitted anxiety.
Keywords: anterior cingulate cortex; anxiety; authentic; maternal separation; parvalbumin interneurons; transmit.
Copyright © 2023 the authors.
Figures





References
-
- Baracz SJ, Everett NA, Robinson KJ, Campbell GR, Cornish JL (2020) Maternal separation changes maternal care, anxiety-like behaviour and expression of paraventricular oxytocin and corticotrophin-releasing factor immunoreactivity in lactating rats. J Neuroendocrinol 32:e12861. 10.1111/jne.12861 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
