Light-Driven Enantioselective Carbene-Catalyzed Radical-Radical Coupling
- PMID: 37845183
- PMCID: PMC10841513
- DOI: 10.1002/anie.202312829
Light-Driven Enantioselective Carbene-Catalyzed Radical-Radical Coupling
Abstract
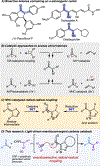
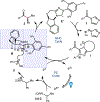
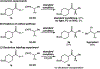
An enantioselective carbene-catalyzed radical-radical coupling of acyl imidazoles and racemic Hantzsch esters is disclosed. This method involves the coupling of an N-heterocyclic carbene-derived ketyl radical and a secondary sp3 -carbon radical and allows access to chiral α-aryl aliphatic ketones in moderate-to-good yields and enantioselectivities without any competitive epimerization. The utility of this protocol is highlighted by the late-stage functionalization of various pharmaceutical compounds and is further demonstrated by the transformation of the enantioenriched products to biologically relevant molecules. Computational investigations reveal the N-heterocyclic carbene controls the double-facial selectivity of the ketyl radical and the alkyl radicals, respectively.
Keywords: Asymmetric Catalysis; N-Heterocyclic Carbenes; Organocatalysis; Photoredox Catalysis; Radicals.
© 2023 Wiley-VCH GmbH.
Figures


References
-
- Corey EJ, Kurti L, Enantioselective Chemical Synthesis: Methods, Logic, and Practice, Elsevier, 2013.
Grants and funding
LinkOut - more resources
Full Text Sources

