Pyrroline-5-carboxylate reductase 1 reprograms proline metabolism to drive breast cancer stemness under psychological stress
- PMID: 37845207
- PMCID: PMC10579265
- DOI: 10.1038/s41419-023-06200-5
Pyrroline-5-carboxylate reductase 1 reprograms proline metabolism to drive breast cancer stemness under psychological stress
Abstract
Cancer stem-like cells (CSCs) contribute to cancer metastasis, drug resistance and tumor relapse, yet how amino acid metabolism promotes CSC maintenance remains exclusive. Here, we identify that proline synthetase PYCR1 is critical for breast cancer stemness and tumor growth. Mechanistically, PYCR1-synthesized proline activates cGMP-PKG signaling to enhance cancer stem-like traits. Importantly, cGMP-PKG signaling mediates psychological stress-induced cancer stem-like phenotypes and tumorigenesis. Ablation of PYCR1 markedly reverses psychological stress-induced proline synthesis, cGMP-PKG signaling activation and cancer progression. Clinically, PYCR1 and cGMP-PKG signaling components are highly expressed in breast tumor specimens, conferring poor survival in breast cancer patients. Targeting proline metabolism or cGMP-PKG signaling pathway provides a potential therapeutic strategy for breast patients undergoing psychological stress. Collectively, our findings unveil that PYCR1-enhanced proline synthesis displays a critical role in maintaining breast cancer stemness.
© 2023. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures

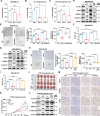

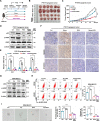
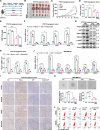

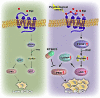
References
-
- Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9:176–98.. doi: 10.1158/2159-8290.CD-18-1177. - DOI - PMC - PubMed
-
- Lee KM, Giltnane JM, Balko JM, Schwarz LJ, Guerrero-Zotano AL, Hutchinson KE, et al. MYC and MCL1 Cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab. 2017;26:633–47.e7. doi: 10.1016/j.cmet.2017.09.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

