A human lung alveolus-on-a-chip model of acute radiation-induced lung injury
- PMID: 37845224
- PMCID: PMC10579267
- DOI: 10.1038/s41467-023-42171-z
A human lung alveolus-on-a-chip model of acute radiation-induced lung injury
Abstract
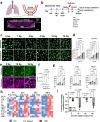
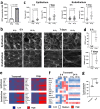
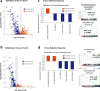
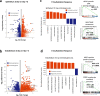
Acute exposure to high-dose gamma radiation due to radiological disasters or cancer radiotherapy can result in radiation-induced lung injury (RILI), characterized by acute pneumonitis and subsequent lung fibrosis. A microfluidic organ-on-a-chip lined by human lung alveolar epithelium interfaced with pulmonary endothelium (Lung Alveolus Chip) is used to model acute RILI in vitro. Both lung epithelium and endothelium exhibit DNA damage, cellular hypertrophy, upregulation of inflammatory cytokines, and loss of barrier function within 6 h of radiation exposure, although greater damage is observed in the endothelium. The radiation dose sensitivity observed on-chip is more like the human lung than animal preclinical models. The Alveolus Chip is also used to evaluate the potential ability of two drugs - lovastatin and prednisolone - to suppress the effects of acute RILI. These data demonstrate that the Lung Alveolus Chip provides a human relevant alternative for studying the molecular basis of acute RILI and may be useful for evaluation of new radiation countermeasure therapeutics.
© 2023. Springer Nature Limited.
Conflict of interest statement
D.E.I. is a founder, board member, and chairs the SAB of Emulate Inc., in which he also holds equity. The remaining authors declare no competing interests.
Figures

References
-
- Society, A., Cancer treatment & survivorship facts & figures 2019–2021. 2019, American Cancer Society Atlanta.
-
- Bledsoe TJ, Nath SK, Decker RH. Radiation pneumonitis. Clinics in chest medicine. 2017;38:201–208. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

