Unexpected gender differences in progressive supranuclear palsy reveal efficacy for davunetide in women
- PMID: 37845254
- PMCID: PMC10579238
- DOI: 10.1038/s41398-023-02618-9
Unexpected gender differences in progressive supranuclear palsy reveal efficacy for davunetide in women
Abstract
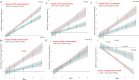
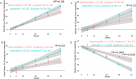
Progressive supranuclear palsy (PSP) is a pure tauopathy, implicating davunetide, enhancing Tau-microtubule interaction, as an ideal drug candidate. However, pooling patient data irrespective of sex concluded no efficacy. Here, analyzing sex-dependency in a 52 week-long- PSP clinical trial (involving over 200 patients) demonstrated clear baseline differences in brain ventricular volumes, a secondary endpoint. Dramatic baseline ventricular volume-dependent/volume increase correlations were observed in 52-week-placebo-treated females (r = 0.74, P = 2.36-9), whereas davunetide-treated females (like males) revealed no such effects. Assessment of primary endpoints, by the PSP Rating Scale (PSPRS) and markedly more so by the Schwab and England Activities of Daily Living (SEADL) scale, showed significantly faster deterioration in females, starting at trial week 13 (P = 0.01, and correlating with most other endpoints by week 52). Twice daily davunetide treatments slowed female disease progression and revealed significant protection according to the SEADL scale as early as at 39 weeks (P = 0.008), as well as protection of the bulbar and limb motor domains considered by the PSPRS, including speaking and swallowing difficulties caused by brain damage, and deterioration of fine motor skills, respectably (P = 0.01), at 52 weeks. Furthermore, at 52 weeks of trial, the exploratory Geriatric Depression Scale (GDS) significantly correlated with the SEADL scale deterioration in the female placebo group and demonstrated davunetide-mediated protection of females. Female-specific davunetide-mediated protection of ventricular volume corresponded to clinical efficacy. Together with the significantly slower disease progression seen in men, the results reveal sex-based drug efficacy differences, demonstrating the neuroprotective and disease-modifying impact of davunetide treatment for female PSP patients.
© 2023. Springer Nature Limited.
Conflict of interest statement
Sex-dependent use of davunetide for PSP and other conditions is under international patent application No. PCT PCT/IL2022/051314 (all authors are listed as inventors).
Figures



References
-
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–93. - PubMed
-
- Amram N, Hacohen-Kleiman G, Sragovich S, Malishkevich A, Katz J, Touloumi O, et al. Sexual divergence in microtubule function: the novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol Psychiatry. 2016;21:1467–76. - PubMed
-
- Karmon G, Sragovich S, Hacohen-Kleiman G, Ben-Horin-Hazak I, Kasparek P, Schuster B, et al. Novel ADNP syndrome mice reveal dramatic sex-specific peripheral gene expression with brain synaptic and Tau pathologies. Biol Psychiatry. 2022;92:81–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

