Follow-up of the GHSG HD16 trial of PET-guided treatment in early-stage favorable Hodgkin lymphoma
- PMID: 37845285
- PMCID: PMC10776396
- DOI: 10.1038/s41375-023-02064-y
Follow-up of the GHSG HD16 trial of PET-guided treatment in early-stage favorable Hodgkin lymphoma
Abstract
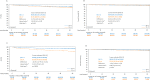
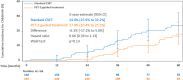
The primary analysis of the GHSG HD16 trial indicated a significant loss of tumor control with PET-guided omission of radiotherapy (RT) in patients with early-stage favorable Hodgkin lymphoma (HL). This analysis reports long-term outcomes. Overall, 1150 patients aged 18-75 years with newly diagnosed early-stage favorable HL were randomized between standard combined-modality treatment (CMT) (2x ABVD followed by PET/CT [PET-2] and 20 Gy involved-field RT) and PET-2-guided treatment omitting RT in case of PET-2 negativity (Deauville score [DS] < 3). The study aimed at excluding inferiority of PET-2-guided treatment and assessing the prognostic impact of PET-2 in patients receiving CMT. At a median follow-up of 64 months, PET-2-negative patients had a 5-year progression-free survival (PFS) of 94.2% after CMT (n = 328) and 86.7% after ABVD alone (n = 300; HR = 2.05 [1.20-3.51]; p = 0.0072). 5-year OS was 98.3% and 98.8%, respectively (p = 0.14); 4/12 documented deaths were caused by second primary malignancies and only one by HL. Among patients assigned to CMT, 5-year PFS was better in PET-2-negative (n = 353; 94.0%) than in PET-2-positive patients (n = 340; 90.3%; p = 0.012). The difference was more pronounced when using DS4 as cut-off (DS 1-3: n = 571; 94.0% vs. DS ≥ 4: n = 122; 83.6%; p < 0.0001). Taken together, CMT should be considered standard treatment for early-stage favorable HL irrespective of the PET-2-result.
© 2023. The Author(s).
Conflict of interest statement
MF: Employment/leadership position (University Hospital of Cologne, Head of the GHSG Trial Coordination Centre), honorarium (Celgene, BMS, Takeda, Affimed, Lukon, Janssen). ASJ: No personal disclosures. HK: No personal disclosures. CK: No personal disclosures. TP: No personal disclosures. PJB: Personal disclosures regarding consulting (BeiGene, Takeda), honorarium (BeiGene, BMS, MSD Stemline, Takeda), financing of scientific studies (BeiGene, BMS, MSD, Takeda), other financial relations (Celgene (travel)). MS: No personal disclosures. MV: No personal disclosures. UD: Personal disclosures regarding honorarium (Amgen, Avencell (DSMB activities)). JM: Personal disclosures regarding consulting (MSD) and other financial relations (Travel support Takeda, MSD, BMS). CB: No personal disclosures. VD: No personal disclosures. AR: No personal disclosures. BvT: Personal disclosures regarding consulting (Allogene, BMS/Celgene, Cerus, Incyte, IQVIA, Gilead Kite, Miltenyi, Novartis, Noscendo, Pentixapharm, Roche, Amgen, Pfizer, Takeda, Merck Sharp & Dohme, and Gilead Kite), honorarium (AstraZeneca, BMS, Incyte, Novartis, Roche Pharma AG, Takeda, and Merck Sharp & Dohme), financing of scientific studies (Novartis (Inst), Merck Sharp & Dohme (Inst), and Takeda (Inst)) and other financial relations (Travel support and congress fees: AbbVie, AstraZeneca, Gilead Kite, Merck Sharp & Dohme, Roche, Takeda, and Novartis). MD: No personal disclosures. DAE: Personal disclosures regarding honorarium (Sanofi-Genzyme, Takeda).
Figures


References
-
- Behringer K, Goergen H, Hitz F, Zijlstra J, Greil R, Markova J, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favorable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomized, non-inferiority trial. Lancet. 2015;385:1418–27. doi: 10.1016/S0140-6736(14)61469-0. - DOI - PubMed
-
- Sasse S, Brockelmann PJ, Goergen H, Pluetschow A, Mueller H, Kreissl S, et al. Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: updated analyses of the German Hodgkin Study Group HD7, HD8, HD10, and HD11 trials. J Clin Oncol. 2017;35:1999–2007. doi: 10.1200/JCO.2016.70.9410. - DOI - PubMed

