Endotoxin removal therapy with Polymyxin B immobilized fiber column: a single center experience from EUPHAS2 registry
- PMID: 37845296
- PMCID: PMC10579294
- DOI: 10.1038/s41598-023-44850-9
Endotoxin removal therapy with Polymyxin B immobilized fiber column: a single center experience from EUPHAS2 registry
Abstract
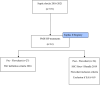
Although the precise clinical indication for initiation of PMX-HA is widely debated in the literature, a proper patient selection and timing of treatment delivery might play a critical role in the clinical course of a specific subphenotype of septic shock (endotoxic shock). In light of this view, since 2019, we have introduced in our clinical practice a diagnostic-therapeutic flowchart to select patients that can benefit the most from the treatment proposed. In addition, we reported in this study our experience of PMX-HA in a cohort of critically ill patients admitted to our intensive care unit (ICU). We analyzed a single centre, retrospective, observational web-based database (extracted from the EUPHAS2 registry) of critically ill patients admitted to the ICU between January 2016 and May 2021 who were affected by endotoxic shock. Patients were divided according to the diagnostic-therapeutic flowchart in two groups: Pre-Flowchart (Pre-F) and Post-Flowchart (Post-F). From January 2016 to May 2021, 61 patients were treated with PMX-HA out of 531 patients diagnosed with septic shock and of these, fifty patients (82%) developed AKI during their ICU stay. The most common source of infection was secondary peritonitis (36%), followed by community-acquired pneumonia (29%). Fifty-five (90%) out of 61 patients received a second PMX-HA treatment, with a statistically significant difference between the two groups (78% of the Pre-F vs. 100% of the Post-F group, p = 0.005). In both groups, between T0 and T120, the Endotoxin Activity Assay (EAA) decreased, while the SOFA score, mean arterial pressure (MAP), and Vasoactive Inotropic Score (VIS) improved with no statistically significant difference. Furthermore, when performing a propensity score matching analysis to compare mortality between the two groups, statistically significant lower ICU and 90-day mortalities were observed in the Post-F group [p = 0.016]. Although in this experienced centre data registry, PMX-HA was associated with organ function recovery, hemodynamic improvement, and current EAA level reduction in critically ill patients with endotoxic shock. Following propensity score-matched analysis, ICU mortality and 90-day mortalities were lower in the diagnostic-therapeutic flowchart group when considering two temporal groups based on strict patient selection criteria and timing to achieve PMX. Further Randomised Control Trials focused on centre selection, adequate training and a flowchart of action when assessing extracorporeal blood purification use should be performed.
© 2023. Springer Nature Limited.
Conflict of interest statement
Prof. Claudio Ronco in the last 3 years has been consultant, medical advisor or part of the speaker bureau receiving fees from the following companies: Asahi Medical, Aferetica, Baxter, B. Braun, Biomerieux, Bioporto, Cytosorbents, ESTOR, Fresenius Medical Care, GE Healthcare, Kaneka, Medica, Medtronic- Bellco, Nipro, Spectral, Toray, Jafron. The other authors have no financial conflicts of interest related to this study.
Figures
References
-
- Tariverdian, T., Zarintaj, P., Milan, P. B., et al. Nanoengineered biomaterials for kidney regeneration. In Nanoengineered Biomaterials for Regenerative Medicine, 325–344 (2019). 10.1016/B978-0-12-813355-2.00014-4.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources