MafB regulates NLRP3 inflammasome activation by sustaining p62 expression in macrophages
- PMID: 37845329
- PMCID: PMC10579372
- DOI: 10.1038/s42003-023-05426-5
MafB regulates NLRP3 inflammasome activation by sustaining p62 expression in macrophages
Abstract
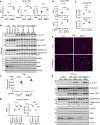
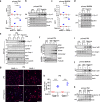
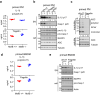
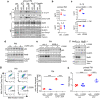
Activation of the NLRP3 inflammasome is a two-step process: the priming and the activating. The priming step involves the induction of NLRP3 and pro-IL-1β, while the activating step leads to the full inflammasome activation triggered by a NLRP3 activator. Although mechanisms underlying the NLRP3 inflammasome activation have been increasingly clear, the regulation of this process remains incompletely understood. In this study, we find that LPS and Pseudomonas aeruginosa cause a rapid downregulation in MafB transcription in macrophages, which leads to a quick decline in the level of MafB protein because MafB is short-lived and constantly degraded by the ubiquitin/proteasome system. We find that MafB knockdown or knockout markedly enhances the NLRP3, but not the NLRP1, NLRC4, or AIM2, inflammasome activation in macrophages. Conversely, pharmacological induction of MafB diminishes the NLRP3 inflammasome activation. Mechanistically, we find that MafB sustains the expression of p62, a key mediator of autophagy/mitophagy. We find that MafB inhibits mitochondrial damage, and mitochondrial ROS production and DNA cytoplasmic release. Furthermore, we find that myeloid MafB deficient mice demonstrate increased systemic and lung IL-1β production in response to LPS treatment and P. aeruginosa infection and deficient lung P. aeruginosa clearance in vivo. In conclusion, our study demonstrates that MafB is an important negative regulator of the NLRP3 inflammasome. Our findings suggest that strategies elevating MafB may be effective to treat immune disorders due to excessive activation of the NLRP3 inflammasome.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

