Regulatory controls of duplicated gene expression during fiber development in allotetraploid cotton
- PMID: 37845354
- PMCID: PMC10632151
- DOI: 10.1038/s41588-023-01530-8
Regulatory controls of duplicated gene expression during fiber development in allotetraploid cotton
Abstract
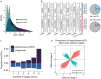
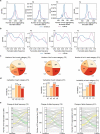
Polyploidy complicates transcriptional regulation and increases phenotypic diversity in organisms. The dynamics of genetic regulation of gene expression between coresident subgenomes in polyploids remains to be understood. Here we document the genetic regulation of fiber development in allotetraploid cotton Gossypium hirsutum by sequencing 376 genomes and 2,215 time-series transcriptomes. We characterize 1,258 genes comprising 36 genetic modules that control staged fiber development and uncover genetic components governing their partitioned expression relative to subgenomic duplicated genes (homoeologs). Only about 30% of fiber quality-related homoeologs show phenotypically favorable allele aggregation in cultivars, highlighting the potential for subgenome additivity in fiber improvement. We envision a genome-enabled breeding strategy, with particular attention to 48 favorable alleles related to fiber phenotypes that have been subjected to purifying selection during domestication. Our work delineates the dynamics of gene regulation during fiber development and highlights the potential of subgenomic coordination underpinning phenotypes in polyploid plants.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

