Development of a mobile laboratory system in hydrogen fuel cell buses and evaluation of the performance for COVID-19 RT-PCR testing
- PMID: 37845364
- PMCID: PMC10579409
- DOI: 10.1038/s41598-023-44925-7
Development of a mobile laboratory system in hydrogen fuel cell buses and evaluation of the performance for COVID-19 RT-PCR testing
Abstract
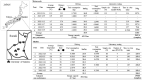
We designed and developed two new types of hydrogen fuel cell (HFC) buses (motorcoach and minibus) with a mobile laboratory system. Feasibility studies have been performed for mobile laboratory testing, particularly for the laboratory performance of COVID-19 RT-PCR (PCR). We evaluated the driving range capability, PCR sample size capacity, turnaround time (TAT), and analytical performance for the detection of SARS-CoV-2. Saliva samples were used for the current study, and the analytical performance was compared with that of the reference PCR. The estimated driving range and sample size capacity of the HFC and HFC minibus were 432 km and 2847 samples, respectively, for the HFC motorcoach and 313 km and 1949 samples for the HFC minibus. For the TAT, the median time between sample submission and completion of PCR was 86 min for the motorcoach and 76 min for the minibus, and the median time between sample submission and electronic reporting of the result to each visitor was 182 min for the motorcoach and 194 min for the minibus. A secondary analysis of 1574 HFC mobile laboratory testing samples was conducted, and all negative samples were found to be negative by reference PCR. Furthermore, all samples were confirmed to be positive by reference PCR or other molecular examinations.
© 2023. Springer Nature Limited.
Conflict of interest statement
TOYOBO Co., Ltd., provided lecture fees for Hiromichi Suzuki and advisory fees for Hiromichi Suzuki. Hiromichi Suzuki received advisory fees from PSS. The other authors declare no competing interests.
Figures


Similar articles
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
Clinical Evaluation of BioFire COVID-19 Test, BioFire Respiratory Panel 2.1, and Cepheid Xpert Xpress SARS-CoV-2 Assays for Sample-to-Answer Detection of SARS-CoV-2.Genes (Basel). 2023 Jan 16;14(1):233. doi: 10.3390/genes14010233. Genes (Basel). 2023. PMID: 36672974 Free PMC article.
-
Assessment of the Diagnostic Ability of Four Detection Methods Using Three Sample Types of COVID-19 Patients.Front Cell Infect Microbiol. 2021 Jun 7;11:685640. doi: 10.3389/fcimb.2021.685640. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34164346 Free PMC article.
-
Lab-in-a-van: Rapid SARS-CoV-2 testing response with a mobile laboratory.EBioMedicine. 2022 May;79:103983. doi: 10.1016/j.ebiom.2022.103983. Epub 2022 Apr 8. EBioMedicine. 2022. PMID: 35405388 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
Cited by
-
Existing operational standards for field deployments of rapid response mobile laboratories: a scoping review.Front Public Health. 2024 Nov 13;12:1455738. doi: 10.3389/fpubh.2024.1455738. eCollection 2024. Front Public Health. 2024. PMID: 39606067 Free PMC article.
-
Transitioning towards hydrogen powered seaport yard cranes for low carbon transformation based on a multiple factor real option model.Sci Rep. 2025 May 20;15(1):17524. doi: 10.1038/s41598-025-00894-7. Sci Rep. 2025. PMID: 40394085 Free PMC article.
-
Validation of a mobile clinical pathology laboratory for canine hematology and biochemistry.BMC Vet Res. 2025 Apr 22;21(1):283. doi: 10.1186/s12917-025-04601-6. BMC Vet Res. 2025. PMID: 40264110 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

