Neoplasia risk in patients with Lynch syndrome treated with immune checkpoint blockade
- PMID: 37845474
- PMCID: PMC10870255
- DOI: 10.1038/s41591-023-02544-9
Neoplasia risk in patients with Lynch syndrome treated with immune checkpoint blockade
Abstract
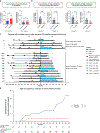
Metastatic and localized mismatch repair-deficient (dMMR) tumors are exquisitely sensitive to immune checkpoint blockade (ICB). The ability of ICB to prevent dMMR malignant or pre-malignant neoplasia development in patients with Lynch syndrome (LS) is unknown. Of 172 cancer-affected patients with LS who had received ≥1 ICB cycles, 21 (12%) developed subsequent malignancies after ICB exposure, 91% (29/32) of which were dMMR, with median time to development of 21 months (interquartile range, 6-38). Twenty-four of 61 (39%) ICB-treated patients who subsequently underwent surveillance colonoscopy had premalignant polyps. Within matched pre-ICB and post-ICB follow-up periods, the overall rate of tumor development was unchanged; however, on subgroup analysis, a decreased incidence of post-ICB visceral tumors was observed. These data suggest that ICB treatment of LS-associated tumors does not eliminate risk of new neoplasia development, and LS-specific surveillance strategies should continue. These data have implications for immunopreventative strategies and provide insight into the immunobiology of dMMR tumors.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests:
Emily Harrold (EH) has received funding from the Conquer Cancer ASCO Foundation. She also served as a consultant to Pfizer Ireland on one occasion in 2021. She reports education grants from Merck and Amgen to attend GI ASCO in 2020.
Benoit Rousseau (BR) serves as a consultant for Neophore and has a patent: Methods and composition for cancer immunotherapy.
Fergus Keane (FR) has received funding from the Conquer Cancer ASCO Foundation.
Andrea Cercek (AC) serves as a consultant for Bayer, GlaxoSmithKline, Janssen Biotech, Merck, Seagen Inc, and receives research funding from GlaxoSmithKline, Inspirna, Seagen Inc.
Rona Yaeger (RY) serves as a consultant for Array BioPharma/ Pfizer, Amgen, Mirati Therapeutics, and Natera, and receives research funding to her institution from Pfizer, Boehringer Ingelheim, Mirati Therapeutics and Daiichi Sankyo
Dana Rathkopf (DR) is an uncompensated Advisor/Steering Committee member and Research Support (PI): Janssen, Astra Zeneca, Bayer, Myovant, Genentech, Promontory, BMS/Celgene
Neil Segal (NHS) serves as a consultant for ABL Bio, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Immunocore, Novartis, Psioxus, Puretech, Revitope, Roche/ Genentech and Numab and receives grant/contracts from: AstraZeneca, Bristol Myers Squibb Company, Immunocore, Merck, Pfizer, Puretech, Regeneron Pharmaceuticals Inc., Roche/ Genentech and Agenus.
Eileen M. O’Reilly (EO) Receives research from Genentech/Roche, Celgene/BMS, BioNTech, AstraZeneca, Arcus, Elicio, Parker Institute, NIH/NCI, and serves as a consultant for: Cytomx Therapeutics (DSMB), Rafael Therapeutics (DSMB), Seagen, Boehringer Ingelheim, BioNTech, Ipsen, Merck, IDEAYA, Silenseed, Novartis, AstraZeneca, BioSapien, Astellas, Thetis, Autem, Novocure, Neogene, BMS, ZielBio, Merus, Tempus, Fibrogen. An immediate family member serves as a consultant for Agios, Genentech-Roche, Eisai and Servier.
Diane Reidy (DR) Receives research funds from Merck, Novartis, and Ipsen, and is on the Scientific Advisory Board for Chiasma, Novartis, and Advanced Accelerator Applications (AAA).
Yelena Y.Janjigian (YYJ) Receives research funding from Bayer, Bristol-Myers Squibb, Cycle for Survival, Department of Defense, Eli Lilly, Fred’s Team, Genentech/Roche, Merck, NCI, RGENIX, serves on the advisory boards/is a consultant for Amerisource Bergen, Arcus Biosciences, Astra Zeneca, Basilea Pharmaceutica, Bayer, Bristol, Myers Squibb, Daiichi-Sankyo, Eli Lilly, Geneos Therapeutics, GlaxoSmithKline, Imedex, Imugene, Lynx Health, Merck, Merck Serono, Mersana Therapeutics, Michael J. Hennessy Associates, Paradigm Medical Communications, PeerView Institute, Pfizer, Research to Practice, RGENIX, Seagen, Silverback Therapeutics, Zymeworks Inc., and has stock options in RGENIX.
Yonina R. Murciano-Goroff (YRMG) reports travel, accommodation, and expenses from AstraZeneca and LOXO Oncology/ Eli Lilly. She acknowledges honoraria from Virology Education and Projects in Knowledge (for a CME program funded by an educational grant from Amgen). She acknowledges associated research funding to the institution from Mirati Therapetuics, Loxo Oncology at Eli Lilly, Elucida Oncology, Taiho Oncology, Hengrui USA, Ltd/ Jiangsu Hengrui Pharmaceuticals, Luzsana Biotechnology, Endeavor Biomedicines, and AbbVie. She is an employee of Memorial Sloan Kettering Cancer Center, which has an institutional interest in Elucida. She acknowledges royalties from Rutgers University Press and Wolters Kluwer. She acknowledges food/beverages from Endeavor Biomedicines. Y.R. Murciano-Goroff acknowledges receipt of training through an institutional K30 grant from the NIH (CTSA UL1TR00457). She has received funding from a Kristina M. Day Young Investigator Award from Conquer Cancer, the ASCO Foundation, endowed by Dr. Charles M. Baum and Carol A. Baum. She is also funded by the Fiona and Stanley Druckenmiller Center for Lung Cancer Research, the Andrew Sabin Family Foundation, the Society for MSK, and a Paul Calabresi Career Development Award for Clinical Oncology (NIH/NCI K12 CA184746).
Ying L. Liu (YLL) reports research funding from AstraZeneca, GSK, and Repare Therapeutics outside this work.
Jonathan E. Rosenberg (JER) has received research support for clinical trials from Bayer, Seagen, Astellas, AstraZeneca, and Roche/Genentech. He has served as an advisor or consultant to Bayer, Seagen, Astellas, AstraZeneca, Roche/Genentech, BMS, Merck, Pfizer, Boehringer Ingelheim, GSK, Janssen, Mirati, EMD-Serono, Gilead, Alligator Biosciences, Eli Lilly, Tyra Biosciences, Infinity, IMVax, Aadi, Century Therapeutics, Emergence Therapeutics, Hengrui
Martin R Weiser (MRW) – Copyright: participate on online tumor board. Licensee (Precisa), Editor of Colorectal Section. Licensee (UpToDate)
Anthony M. Rossi (AMR) serves as Regeneron: Consultant; Evolve CME: Consultant; Almirall: Consultant; Mavig: Travel; Merz: Consultant; Dynamed: Consultant; Canfield Scientific: Consultant; AllerganInc: Advisory Board; Evolus: Consultant; Biofrontera: Consulatant; QuantiaMD: Consultant; Lam Therapeutics; Consultant; Cutera: Consultant; Skinfix, advisor; L’oreal, travel, DAR companies: Founder; Skinpass Board. AMR received research/study funding from ASLMS: A Ward Memorial Research Grant, Skin Cancer Foundation, Regen, LeoPharma, Biofrontera and serves as Editorial Board: Lasers in Surgery and Medicine; CUTIS, Editorial Board: Journal of the American Academy of Dermatology (JAAD); Dermatologic Surgery, Board Member: ASDS, Committee Member and / or Chair: AAD; ASDS; ASLM.
Kenneth Offit (KO) is a founder (uncompenstated; shares not alloted) of AnaNeo Therapeutics, Inc.
Patents, Royalties, Other Intellectual Property: Diagnosis and treatment of ERCC3-mutant cancer; inventors: Joseph Vijai, Sabine Topka, Kenneth Offit; US National Stage Patent Application No.: 16/493,214; filing date: September 11, 2019 (Inst)
Michael F. Berger (MFB) serves as a consultant for Eli Lilly and Astra Zeneca, and receives research support from Boundless Bio.
David B. Solit (DBS) has served as a consultant for/received honorarium from Pfizer, Loxo/Lilly Oncology, Vividion Therapeutics, Scorpion Therapeutics, FORE Therapeutics, Fog Pharma, Elsie Biotechnologies, and BridgeBio
Leonard Saltz (LS) serves as consultant and is a member of the Scientific Advisory Board for Genor Biopharma Ltd.
Jinru Shia (JS) serves as a consultant for Paige AI.
Luis Diaz (LD) is a member of the board of directors of Jounce Therapeutics and Epitope. He is a compensated consultant to PetDx, Innovatus CP, Se’er, Delfi, Blackstone, Kinnate and Neophore. LD is an inventor of multiple licensed patents related to technology for circulating tumor DNA analyses and mismatch repair deficiency for diagnosis and therapy. Some of these licenses and relationships are associated with equity or royalty payments to the inventors. He holds equity in Epitope, Jounce Therapeutics, PetDx, Se’er, Delfi, Kinnate and Neophore. He divested his equity in Personal Genome Diagnostics to LabCorp in February 2022 and divested his equity in Thrive Earlier Detection to Exact Biosciences in January 2021. His spouse holds equity in Amgen. The terms of all these arrangements are being managed by Memorial Sloan Kettering in accordance with their conflict-of-interest policy.
Zsofia K. Stadler’s (ZKS) immediate family member serves as a consultant in Ophthalmology for Adverum, Genentech, Neurogene, Novartis, Optos Plc, Outlook Therapeutics, and Regeneron outside the submitted work. ZKS serves as an Associate Editor for
The remaining authors have no competing interests.
Figures







References
-
- Cercek Andrea, et al. New England Journal of Medicine (2022).
-
- Chalabi Myriam, et al. Nature medicine 26.4 (2020): 566–576. - PubMed
Methods Only References:
-
- Shia Jinru. “The diversity of tumours with microsatellite instability: molecular mechanisms and impact upon microsatellite instability testing and mismatch repair protein immunohistochemistry.” Histopathology 78.4 (2021): 485–497. - PubMed
-
- Ziegler John, et al. “MiMSI-a deep multiple instance learning framework improves microsatellite instability detection from tumor next-generation sequencing.” bioRxiv (2020).
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

