cAMP-mediated upregulation of HCN channels in VTA dopamine neurons promotes cocaine reinforcement
- PMID: 37845497
- PMCID: PMC10730389
- DOI: 10.1038/s41380-023-02290-x
cAMP-mediated upregulation of HCN channels in VTA dopamine neurons promotes cocaine reinforcement
Abstract
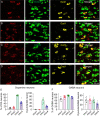
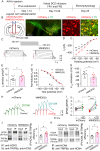
Chronic cocaine exposure induces enduring neuroadaptations that facilitate motivated drug taking. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are known to modulate neuronal firing and pacemaker activity in ventral tegmental area (VTA) dopamine neurons. However, it remained unknown whether cocaine self-administration affects HCN channel function and whether HCN channel activity modulates motivated drug taking. We report that rat VTA dopamine neurons predominantly express Hcn3-4 mRNA, while VTA GABA neurons express Hcn1-4 mRNA. Both neuronal types display similar hyperpolarization-activated currents (Ih), which are facilitated by acute increases in cAMP. Acute cocaine application decreases voltage-dependent activation of Ih in VTA dopamine neurons, but not in GABA neurons. Unexpectedly, chronic cocaine self-administration results in enhanced Ih selectively in VTA dopamine neurons. This differential modulation of Ih currents is likely mediated by a D2 autoreceptor-induced decrease in cAMP as D2 (Drd2) mRNA is predominantly expressed in dopamine neurons, whereas D1 (Drd1) mRNA is barely detectable in the VTA. Moreover, chronically decreased cAMP via Gi-DREADD stimulation leads to an increase in Ih in VTA dopamine neurons and enhanced binding of HCN3/HCN4 with tetratricopeptide repeat-containing Rab8b-interacting protein (TRIP8b), an auxiliary subunit that is known to facilitate HCN channel surface trafficking. Finally, we show that systemic injection and intra-VTA infusion of the HCN blocker ivabradine reduces cocaine self-administration under a progressive ratio schedule and produces a downward shift of the cocaine dose-response curve. Our results suggest that cocaine self-administration induces an upregulation of Ih in VTA dopamine neurons, while HCN inhibition reduces the motivation for cocaine intake.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–80. - PubMed
-
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411:805–10. - PubMed
-
- Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979;280:235–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

