Antimicrobial peptides modulate lung injury by altering the intestinal microbiota
- PMID: 37845716
- PMCID: PMC10578018
- DOI: 10.1186/s40168-023-01673-0
Antimicrobial peptides modulate lung injury by altering the intestinal microbiota
Abstract
Background: Mammalian mucosal barriers secrete antimicrobial peptides (AMPs) as critical, host-derived regulators of the microbiota. However, mechanisms that support microbiota homeostasis in response to inflammatory stimuli, such as supraphysiologic oxygen, remain unclear.
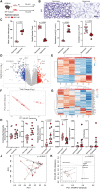
Results: We show that supraphysiologic oxygen exposure to neonatal mice, or direct exposure of intestinal organoids to supraphysiologic oxygen, suppresses the intestinal expression of AMPs and alters intestinal microbiota composition. Oral supplementation of the prototypical AMP lysozyme to hyperoxia-exposed neonatal mice reduced hyperoxia-induced alterations in their microbiota and was associated with decreased lung injury.
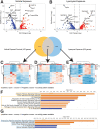
Conclusions: Our results identify a gut-lung axis driven by intestinal AMP expression and mediated by the intestinal microbiota that is linked to lung injury in newborns. Together, these data support that intestinal AMPs modulate lung injury and repair. Video Abstract.
Keywords: Bronchopulmonary dysplasia; Chronic lung disease; Gut-lung axis; Lysozyme; Microbiome; Neonatal lung injury; Neonate; Post-prematurity lung disease.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
CL is the founder and CEO of Alveolus Bio and Resbiotic, Inc., NA and KW are advisors, and TN is now an employee.
Figures


Update of
-
Antimicrobial peptides modulate lung injury by altering the intestinal microbiota.bioRxiv [Preprint]. 2023 Mar 15:2023.03.14.529700. doi: 10.1101/2023.03.14.529700. bioRxiv. 2023. Update in: Microbiome. 2023 Oct 16;11(1):226. doi: 10.1186/s40168-023-01673-0. PMID: 36993189 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

