Immunomodulatory effects of inactivated Ligilactobacillus salivarius CECT 9609 on respiratory epithelial cells
- PMID: 37845774
- PMCID: PMC10580541
- DOI: 10.1186/s13567-023-01228-z
Immunomodulatory effects of inactivated Ligilactobacillus salivarius CECT 9609 on respiratory epithelial cells
Abstract
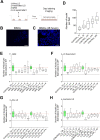
The microbiota in humans and animals play crucial roles in defense against pathogens and offer a promising natural source for immunomodulatory products. However, the development of physiologically relevant model systems and protocols for testing such products remains challenging. In this study, we present an experimental condition where various natural products derived from the registered lactic acid bacteria Ligilactobacillus salivarius CECT 9609, known for their immunomodulatory activity, were tested. These products included live and inactivated bacteria, as well as fermentation products at different concentrations and culture times. Using our established model system, we observed no morphological changes in the airway epithelium upon exposure to Pasteurella multocida, a common respiratory pathogen. However, early molecular changes associated with the innate immune response were detected through transcript analysis. By employing diverse methodologies ranging from microscopy to next-generation sequencing (NGS), we characterized the interaction of these natural products with the airway epithelium and their potential beneficial effects in the presence of P. multocida infection. In particular, our discovery highlights that among all Ligilactobacillus salivarius CECT 9609 products tested, only inactivated cells preserve the conformation and morphology of respiratory epithelial cells, while also reversing or altering the natural immune responses triggered by Pasteurella multocida. These findings lay the groundwork for further exploration into the protective role of these bacteria and their derivatives.
Keywords: Airway epithelial cells; Pasteurella multocida; immunomodulator; lactic acid bacteria; multiciliated cells; secretory cells.
© 2023. L’Institut National de Recherche en Agriculture, Alimentation et Environnement (INRAE).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

