A Single-Arm Phase II Study of Nab-Paclitaxel Plus Gemcitabine and Cisplatin for Locally Advanced or Metastatic Biliary Tract Cancer
- PMID: 37846469
- PMCID: PMC11016659
- DOI: 10.4143/crt.2023.726
A Single-Arm Phase II Study of Nab-Paclitaxel Plus Gemcitabine and Cisplatin for Locally Advanced or Metastatic Biliary Tract Cancer
Abstract
Purpose: Patients with advanced biliary tract cancer (BTC) have a poor survival. We aim to evaluate the efficacy and safety of nab-paclitaxel plus gemcitabine and cisplatin regimen in Chinese advanced BTC patients.
Materials and methods: Eligible patients with locally advanced or metastatic BTC administrated intravenous 100 mg/m2 nab-paclitaxel, 800 mg/m2 gemcitabine, and 25 mg/m2 cisplatin every 3 weeks. The primary endpoint was progression-free survival (PFS). The secondary endpoints included overall survival (OS) and adverse events, while exploratory endpoint was the association of biomarkers with efficacy.
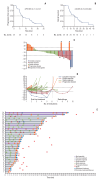
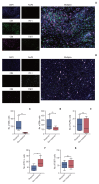
Results: After the median follow-up of 25.0 months, the median PFS and OS of 34 enrolled patients were 7.1 months (95% confidence interval [CI], 5.4 to 13.7) and 16.4 months (95% CI, 10.9 to 23.6), respectively. The most common treatment-related adverse events at ≥ 3 grade were neutropenia (26.5%) and leukopenia (26.5%). Survival analyses demonstrated that carcinoembryonic antigen (CEA) levels could monitor patients' survival outcomes. A significant increase in the number of infiltrating CD4+ cells (p=0.008) and a decrease in programmed death-1-positive (PD-1+) cells (p=0.032) were observed in the response patients.
Conclusion: In advanced BTC patients, nab-paclitaxel plus gemcitabine and cisplatin regimen showed therapeutic potential. Potential prognostic factors of CEA levels, number of CD4+ cells and PD-1+ cells may help us maximize the efficacy benefit.
Keywords: Biliary tract neoplasms; Cisplatin; Gemcitabine; Nab-paclitaxel; Tumor microenvironment.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures

Similar articles
-
Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial.JAMA Oncol. 2019 Jun 1;5(6):824-830. doi: 10.1001/jamaoncol.2019.0270. JAMA Oncol. 2019. PMID: 30998813 Free PMC article. Clinical Trial.
-
Sintilimab plus nab-paclitaxel as second-line treatment for advanced biliary tract cancer: study protocol for an investigator-initiated phase 2 trial (NapaSinti trial).BMC Cancer. 2023 Aug 7;23(1):729. doi: 10.1186/s12885-023-11188-4. BMC Cancer. 2023. PMID: 37550655 Free PMC article.
-
Real-World Outcomes of Gemcitabine, Cisplatin, and Nab-Paclitaxel Chemotherapy Regimen for Advanced Biliary Tract Cancer: A Propensity Score-Matched Analysis.Gut Liver. 2022 Sep 15;16(5):798-805. doi: 10.5009/gnl210346. Epub 2022 Jan 7. Gut Liver. 2022. PMID: 35000934 Free PMC article.
-
Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study.Lancet Gastroenterol Hepatol. 2020 Mar;5(3):285-294. doi: 10.1016/S2468-1253(19)30327-9. Epub 2020 Jan 14. Lancet Gastroenterol Hepatol. 2020. PMID: 31953079 Clinical Trial.
-
Evaluating the Therapeutic Potential of Durvalumab in Adults with Locally Advanced or Metastatic Biliary Tract Cancer: Evidence to Date.Onco Targets Ther. 2024 May 17;17:383-394. doi: 10.2147/OTT.S391707. eCollection 2024. Onco Targets Ther. 2024. PMID: 38774819 Free PMC article. Review.
Cited by
-
Efficacy and biomarker analysis of second-line nab-paclitaxel plus sintilimab in patients with advanced biliary tract cancer.Cancer Sci. 2024 Jul;115(7):2371-2383. doi: 10.1111/cas.16179. Epub 2024 Apr 18. Cancer Sci. 2024. PMID: 38638055 Free PMC article. Clinical Trial.
-
Initial adjustments in the dosage and rest period of gemcitabine plus cisplatin therapy for patients with incurable biliary tract cancer based on baseline estimated glomerular filtration rate (eGFR) values may be crucial for treatment outcomes and the preservation of renal function.J Gastrointest Oncol. 2024 Oct 31;15(5):2277-2285. doi: 10.21037/jgo-24-330. Epub 2024 Oct 24. J Gastrointest Oncol. 2024. PMID: 39554560 Free PMC article.
-
WTAP-mediated N6-methyladenosine modification promotes the inflammation, mitochondrial damage and ferroptosis of kidney tubular epithelial cells in acute kidney injury by regulating LMNB1 expression and activating NF-κB and JAK2/STAT3 pathways.J Bioenerg Biomembr. 2024 Jun;56(3):285-296. doi: 10.1007/s10863-024-10015-0. Epub 2024 Mar 22. J Bioenerg Biomembr. 2024. PMID: 38517565
-
Combined Chemotherapy-Immunotherapy for Advanced Biliary Tract Cancer (BTC): A Clinical, Genomic, and Biomarker Analysis.J Gastrointest Cancer. 2025 Apr 1;56(1):90. doi: 10.1007/s12029-025-01215-x. J Gastrointest Cancer. 2025. PMID: 40167580
References
-
- Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428–44. - PubMed
-
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–80. - PubMed
-
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–89. - PubMed
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

