A Single-Arm Phase II Study of Nab-Paclitaxel Plus Gemcitabine and Cisplatin for Locally Advanced or Metastatic Biliary Tract Cancer
- PMID: 37846469
- PMCID: PMC11016659
- DOI: 10.4143/crt.2023.726
A Single-Arm Phase II Study of Nab-Paclitaxel Plus Gemcitabine and Cisplatin for Locally Advanced or Metastatic Biliary Tract Cancer
Abstract
Purpose: Patients with advanced biliary tract cancer (BTC) have a poor survival. We aim to evaluate the efficacy and safety of nab-paclitaxel plus gemcitabine and cisplatin regimen in Chinese advanced BTC patients.
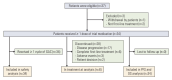
Materials and methods: Eligible patients with locally advanced or metastatic BTC administrated intravenous 100 mg/m2 nab-paclitaxel, 800 mg/m2 gemcitabine, and 25 mg/m2 cisplatin every 3 weeks. The primary endpoint was progression-free survival (PFS). The secondary endpoints included overall survival (OS) and adverse events, while exploratory endpoint was the association of biomarkers with efficacy.
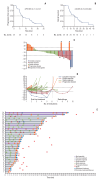
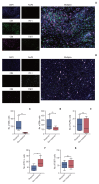
Results: After the median follow-up of 25.0 months, the median PFS and OS of 34 enrolled patients were 7.1 months (95% confidence interval [CI], 5.4 to 13.7) and 16.4 months (95% CI, 10.9 to 23.6), respectively. The most common treatment-related adverse events at ≥ 3 grade were neutropenia (26.5%) and leukopenia (26.5%). Survival analyses demonstrated that carcinoembryonic antigen (CEA) levels could monitor patients' survival outcomes. A significant increase in the number of infiltrating CD4+ cells (p=0.008) and a decrease in programmed death-1-positive (PD-1+) cells (p=0.032) were observed in the response patients.
Conclusion: In advanced BTC patients, nab-paclitaxel plus gemcitabine and cisplatin regimen showed therapeutic potential. Potential prognostic factors of CEA levels, number of CD4+ cells and PD-1+ cells may help us maximize the efficacy benefit.
Keywords: Biliary tract neoplasms; Cisplatin; Gemcitabine; Nab-paclitaxel; Tumor microenvironment.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures

References
-
- Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428–44. - PubMed
-
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–80. - PubMed
-
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–89. - PubMed
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

