CACNA1D Gene Polymorphisms Associate With Increased Blood Pressure and Salt Sensitivity of Blood Pressure in White Individuals
- PMID: 37846579
- PMCID: PMC10843263
- DOI: 10.1161/HYPERTENSIONAHA.123.21229
CACNA1D Gene Polymorphisms Associate With Increased Blood Pressure and Salt Sensitivity of Blood Pressure in White Individuals
Abstract
Background: Disease-causing mutations in CACNA1D gene occur in aldosterone-producing adenomas and familial hyperaldosteronism. We determined whether single nucleotide polymorphisms in CACNA1D gene associate with higher aldosterone resulting in salt sensitivity of blood pressure (BP) and increased BP in men and women.
Methods: Data were obtained from the HyperPATH (International Hypertension Pathotypes) cohort, where participants completed a cross-over intervention of liberal and restricted sodium diets. Multi-Ethnic Genotyping Array identified 104 CACNA1D single nucleotide polymorphisms that met quality control. Single nucleotide polymorphism is rs7612148 strongly associated with systolic BP and was selected for study in 521 White participants in 3 scenarios ([1] hypertensives; [2] normotensives; [3] total population=hypertensives+normotensives) using multivariate regression analysis.
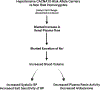
Results: In the total population and hypertensives, but not normotensives, risk allele carriers (CC, GC), as compared with nonrisk allele homozygotes (GG), exhibited higher salt sensitivity of BP and, on liberal sodium diet, higher systolic BP, lower baseline and angiotensin II-stimulated aldosterone, and lower plasma renin activity. On restricted sodium diet, BP was similar across genotypes, suggesting sodium restriction corrected/neutralized the genotype effect on BP. Because increased aldosterone did not seem to drive the increased salt sensitivity of BP and increased BP on liberal sodium diet, we assessed renal plasma flow. Renal plasma flow increase from restricted to liberal sodium diets was blunted in risk allele homozygotes in the total population and in hypertensives. A replication study in another cohort HyperPATH B (International Hypertension Pathotypes Cohort B) confirmed BP-genotype associations.
Conclusions: CACNA1D rs7612148 risk allele associated with increased BP and salt sensitivity of BP, likely due to an impaired ability to increase renal plasma flow in response to a liberal sodium diet and not to excess aldosterone.
Keywords: aldosterone; allele; blood pressure; renal plasma flow.
Conflict of interest statement
Figures

Similar articles
-
Aldosterone synthase gene (CYP11B2) C-344T polymorphism in Caucasians from the Berlin Salt-Sensitivity Trial (BeSST).J Hypertens. 1999 Nov;17(11):1563-7. doi: 10.1097/00004872-199917110-00009. J Hypertens. 1999. PMID: 10608469 Clinical Trial.
-
Polymorphisms in the serum- and glucocorticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake.J Hum Hypertens. 2013 Mar;27(3):176-80. doi: 10.1038/jhh.2012.22. Epub 2012 May 31. J Hum Hypertens. 2013. PMID: 22648267 Free PMC article.
-
Resequencing Study Identifies Rare Renin-Angiotensin-Aldosterone System Variants Associated With Blood Pressure Salt-Sensitivity: The GenSalt Study.Am J Hypertens. 2017 May 1;30(5):495-501. doi: 10.1093/ajh/hpx004. Am J Hypertens. 2017. PMID: 28199472 Free PMC article.
-
Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride.Cochrane Database Syst Rev. 2020 Dec 12;12(12):CD004022. doi: 10.1002/14651858.CD004022.pub5. Cochrane Database Syst Rev. 2020. PMID: 33314019 Free PMC article.
-
Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension.Adv Exp Med Biol. 2017;956:61-84. doi: 10.1007/5584_2016_147. Adv Exp Med Biol. 2017. PMID: 27757935 Review.
Cited by
-
Effects of Cacna1d D307G Mutation on Blood Pressure and Kidney Function in Rats with Salt Loading.Kidney Blood Press Res. 2025;50(1):46-60. doi: 10.1159/000542828. Epub 2024 Dec 2. Kidney Blood Press Res. 2025. PMID: 39622224 Free PMC article.
-
Transcriptomics of SGLT2-positive early proximal tubule segments in mice: response to type 1 diabetes, SGLT1/2 inhibition, or GLP1 receptor agonism.Am J Physiol Renal Physiol. 2025 Jan 1;328(1):F68-F81. doi: 10.1152/ajprenal.00231.2024. Epub 2024 Nov 26. Am J Physiol Renal Physiol. 2025. PMID: 39589189
-
Genome-Wide Approach of Gene-Nutrient Intake Interaction Study for Essential Hypertension in a Large Korean Cohort (KoGES).Nutrients. 2024 Nov 29;16(23):4147. doi: 10.3390/nu16234147. Nutrients. 2024. PMID: 39683541 Free PMC article.
References
-
- Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017;69:1811–1820. doi: 10.1016/j.jacc.2017.01.052 - DOI - PubMed
-
- Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, Beuschlein F, Rossi GP, Nishikawa T, Morganti A, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens. 2020;38:1919–1928. doi: 10.1097/hjh.0000000000002510 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

