An open-label, Phase 3 trial of TAK-003, a live attenuated dengue tetravalent vaccine, in healthy US adults: immunogenicity and safety when administered during the second half of a 24-month shelf-life
- PMID: 37846724
- PMCID: PMC10583633
- DOI: 10.1080/21645515.2023.2254964
An open-label, Phase 3 trial of TAK-003, a live attenuated dengue tetravalent vaccine, in healthy US adults: immunogenicity and safety when administered during the second half of a 24-month shelf-life
Abstract
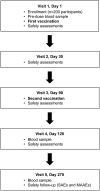
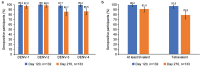
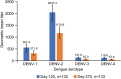
Dengue is caused by a mosquito-transmitted flavivirus. The disease is now endemic to many tropical and subtropical regions, manifesting as approximately 96 million symptomatic cases of dengue each year. Clinical trials have shown TAK-003 (Qdenga®), a live attenuated dengue tetravalent vaccine, to be well-tolerated, immunogenic, and efficacious in adults with no prior exposure to dengue virus infection living in non-endemic regions, as well as in adults and children living in dengue-endemic areas. This open-label, single-arm phase 3 trial (NCT03771963) was conducted in two dengue non-endemic areas of the USA, and it evaluated the immunogenicity and safety of naturally-aged TAK-003 administered to adult participants. Overall, the immunogenicity data from this trial are consistent with those reported from other TAK-003 phase 2 and 3 trials, and the safety data are consistent with the broader integrated safety data analysis. The data show that naturally-aged TAK-003 had a well-tolerated reactogenicity and adverse events profile when administered in the second half of its clinical 24-month shelf-life and that it still elicited an immune response that persisted up to 6 months after the second dose against all four dengue serotypes, with no important safety risks identified during the trial.
Keywords: Dengue fever; dengue vaccine; immunogenicity; live attenuated; safety; shelf-life; tetravalent.
Conflict of interest statement
S.S.P., A.F., F.N., and C.G.-T. are employees of Takeda and own stock/stock options in Takeda. I.L. was an employee of Takeda when this trial was conducted and completed. S.S.P., A.F., F.N., and C.G.-T. report support from Takeda for attending meetings and/or travel, and stock options from Takeda.
Figures

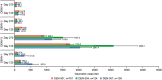
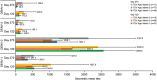
References
-
- López-Medina E, Biswal S, Saez-Llorens X, Borja-Tabora C, Bravo L, Sirivichayakul C, Vargas LM, Alera MT, Velásquez H, Reynales H, et al. Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents 2 years after vaccination. J Infect Dis. 2022;225(9):1521–32. doi:10.1093/infdis/jiaa761. - DOI - PMC - PubMed
-
- World Health Organization . Dengue and severe dengue. 2023. [accessed 2023 Sep 6]. http://www.who.int/mediacentre/factsheets/fs117/en/.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
