Ih current stabilizes excitability in rodent DRG neurons and reverses hyperexcitability in a nociceptive neuron model of inherited neuropathic pain
- PMID: 37846879
- PMCID: PMC10843455
- DOI: 10.1113/JP284999
Ih current stabilizes excitability in rodent DRG neurons and reverses hyperexcitability in a nociceptive neuron model of inherited neuropathic pain
Abstract
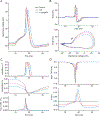
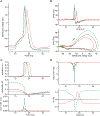
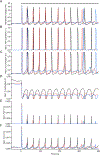
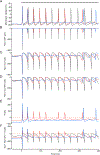
We show here that hyperpolarization-activated current (Ih ) unexpectedly acts to inhibit the activity of dorsal root ganglion (DRG) neurons expressing WT Nav1.7, the largest inward current and primary driver of DRG neuronal firing, and hyperexcitable DRG neurons expressing a gain-of-function Nav1.7 mutation that causes inherited erythromelalgia (IEM), a human genetic model of neuropathic pain. In this study we created a kinetic model of Ih and used it, in combination with dynamic-clamp, to study Ih function in DRG neurons. We show, for the first time, that Ih increases rheobase and reduces the firing probability in small DRG neurons, and demonstrate that the amplitude of subthreshold oscillations is reduced by Ih . Our results show that Ih , due to slow gating, is not deactivated during action potentials (APs) and has a striking damping action, which reverses from depolarizing to hyperpolarizing, close to the threshold for AP generation. Moreover, we show that Ih reverses the hyperexcitability of DRG neurons expressing a gain-of-function Nav1.7 mutation that causes IEM. In the aggregate, our results show that Ih unexpectedly has strikingly different effects in DRG neurons as compared to previously- and well-studied cardiac cells. Within DRG neurons where Nav1.7 is present, Ih reduces depolarizing sodium current inflow due to enhancement of Nav1.7 channel fast inactivation and creates additional damping action by reversal of Ih direction from depolarizing to hyperpolarizing close to the threshold for AP generation. These actions of Ih limit the firing of DRG neurons expressing WT Nav1.7 and reverse the hyperexcitability of DRG neurons expressing a gain-of-function Nav1.7 mutation that causes IEM. KEY POINTS: Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, the molecular determinants of hyperpolarization-activated current (Ih ) have been characterized as a 'pain pacemaker', and thus considered to be a potential molecular target for pain therapeutics. Dorsal root ganglion (DRG) neurons express Nav1.7, a channel that is not present in central neurons or cardiac tissue. Gain-of-function mutations (GOF) of Nav1.7 identified in inherited erythromelalgia (IEM), a human genetic model of neuropathic pain, produce DRG neuron hyperexcitability, which in turn produces severe pain. We found that Ih increases rheobase and reduces firing probability in small DRG neurons expressing WT Nav1.7, and demonstrate that the amplitude of subthreshold oscillations is reduced by Ih . We also demonstrate that Ih reverses the hyperexcitability of DRG neurons expressing a GOF Nav1.7 mutation (L858H) that causes IEM. Our results show that, in contrast to cardiac cells and CNS neurons, Ih acts to stabilize DRG neuron excitability and prevents excessive firing.
Keywords: HCN channels; Hodgkin-Huxley equations; Nav1.7; channelopathy; dynamic-clamp; inherited erythromelalgia; neuropathic pain.
© 2023 The Authors. The Journal of Physiology © 2023 The Physiological Society.
Conflict of interest statement
Competing interests
None of the authors has a conflict of interest
Figures









Similar articles
-
Interplay of Nav1.8 and Nav1.7 channels drives neuronal hyperexcitability in neuropathic pain.J Gen Physiol. 2024 Nov 4;156(11):e202413596. doi: 10.1085/jgp.202413596. Epub 2024 Oct 8. J Gen Physiol. 2024. PMID: 39378238 Free PMC article.
-
Nav1.7-A1632G Mutation from a Family with Inherited Erythromelalgia: Enhanced Firing of Dorsal Root Ganglia Neurons Evoked by Thermal Stimuli.J Neurosci. 2016 Jul 13;36(28):7511-22. doi: 10.1523/JNEUROSCI.0462-16.2016. J Neurosci. 2016. PMID: 27413160 Free PMC article.
-
Dynamic-clamp analysis of wild-type human Nav1.7 and erythromelalgia mutant channel L858H.J Neurophysiol. 2014 Apr;111(7):1429-43. doi: 10.1152/jn.00763.2013. Epub 2014 Jan 8. J Neurophysiol. 2014. PMID: 24401712
-
Familial pain syndromes from mutations of the NaV1.7 sodium channel.Ann N Y Acad Sci. 2010 Jan;1184:196-207. doi: 10.1111/j.1749-6632.2009.05110.x. Ann N Y Acad Sci. 2010. PMID: 20146699 Review.
-
Pain disorders and erythromelalgia caused by voltage-gated sodium channel mutations.Curr Neurol Neurosci Rep. 2012 Feb;12(1):76-83. doi: 10.1007/s11910-011-0233-8. Curr Neurol Neurosci Rep. 2012. PMID: 21984269 Review.
Cited by
-
The Nociceptor Primary Cilium Contributes to Mechanical Nociceptive Threshold and Inflammatory and Neuropathic Pain.J Neurosci. 2024 Nov 20;44(47):e1265242024. doi: 10.1523/JNEUROSCI.1265-24.2024. J Neurosci. 2024. PMID: 39349056 Free PMC article.
-
Sensitization of human and rat nociceptors by low dose morphine is toll-like receptor 4-dependent.Mol Pain. 2024 Jan-Dec;20:17448069241227922. doi: 10.1177/17448069241227922. Mol Pain. 2024. PMID: 38195088 Free PMC article.
-
Construction of a Novel Necroptosis-Related Signature in Rat DRG for Neuropathic Pain.J Inflamm Res. 2025 Jan 6;18:147-165. doi: 10.2147/JIR.S494286. eCollection 2025. J Inflamm Res. 2025. PMID: 39802520 Free PMC article.
-
Morphine acts in vitro to directly prime nociceptors.Mol Pain. 2024 Jan-Dec;20:17448069241260348. doi: 10.1177/17448069241260348. Mol Pain. 2024. PMID: 38828868 Free PMC article.
-
Interplay of Nav1.8 and Nav1.7 channels drives neuronal hyperexcitability in neuropathic pain.J Gen Physiol. 2024 Nov 4;156(11):e202413596. doi: 10.1085/jgp.202413596. Epub 2024 Oct 8. J Gen Physiol. 2024. PMID: 39378238 Free PMC article.
References
-
- Amir R, Liu CN, Kocsis JD & Devor M. (2002). Oscillatory mechanism in primary sensory neurones. Brain 125, 421–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

