Photoinduced Efficient Catalytic α-Alkylation Reactions of Active Methylene and Methine Compounds with Nonactivated Alkenes
- PMID: 37846890
- PMCID: PMC10603815
- DOI: 10.1021/jacs.3c07436
Photoinduced Efficient Catalytic α-Alkylation Reactions of Active Methylene and Methine Compounds with Nonactivated Alkenes
Abstract
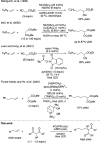
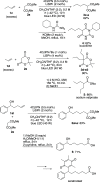
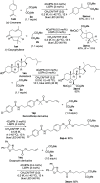
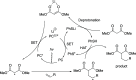
In catalytic α-alkylation reactions of carbonyl compounds, although SN2-type substitution reactions of enolates with alkyl halides are a conventional methodology, addition reactions with alkenes are more desirable because of their atom-economical character; however, reactions with nonactivated alkenes are challenging. Here, we developed highly efficient catalytic α-alkylation reactions of active methylene and methine compounds with nonactivated alkenes such as 1-decene using an organophotocatalyst and lithium thiophenoxide as a Lewis acid/Brønsted base/hydrogen atom transfer (HAT) multifunctional catalyst under blue-light irradiation. The reaction was also performed with a higher degree of efficiency under a continuous-flow system to obtain the products in multigram scales. The present reaction system enables highly efficient and practical α-alkylation reactions of active methylene and methine compounds to be achieved.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Efficient Radical-Mediated Intermolecular α-Alkylation Reactions of Carbonyl Compounds with Nonactivated Alkenes.Chem Asian J. 2024 Jun 17;19(12):e202400319. doi: 10.1002/asia.202400319. Epub 2024 May 14. Chem Asian J. 2024. PMID: 38676345 Review.
-
Visible-Light-Driven Catalytic Alkylation of Reactive Alkyl Nitriles with Nonactivated Alkenes Using an Amine as a Hydrogen Atom Transfer Catalyst.Org Lett. 2025 May 9;27(18):4701-4705. doi: 10.1021/acs.orglett.5c01072. Epub 2025 Apr 30. Org Lett. 2025. PMID: 40302629
-
Direct Asymmetric α-Alkylation of β-Ketocarbonyl Compounds with Simple Olefins by Photoredox-Nickel-Hydrogen Atom Transfer Triple Catalysis.Angew Chem Int Ed Engl. 2025 Apr 17;64(17):e202424915. doi: 10.1002/anie.202424915. Epub 2025 Feb 25. Angew Chem Int Ed Engl. 2025. PMID: 39935403
-
Catalytic alkylation reactions of weakly acidic carbonyl and related compounds using alkenes as electrophiles.Org Biomol Chem. 2018 Aug 22;16(33):5969-5972. doi: 10.1039/c8ob00941d. Org Biomol Chem. 2018. PMID: 29947406
-
Acid-base catalysis of chiral Pd complexes: development of novel catalytic asymmetric reactions and their application to synthesis of drug candidates.Chem Pharm Bull (Tokyo). 2006 Oct;54(10):1351-64. doi: 10.1248/cpb.54.1351. Chem Pharm Bull (Tokyo). 2006. PMID: 17015970 Review.
Cited by
-
Photoredox-Catalyzed Radical Cyclization of Unactivated Alkene-Substituted β-Ketoesters Enabled Asymmetric Total Synthesis of Tricyclic Prostaglandin D2 Metabolite Methyl Ester.JACS Au. 2025 Mar 7;5(3):1367-1375. doi: 10.1021/jacsau.4c01268. eCollection 2025 Mar 24. JACS Au. 2025. PMID: 40151232 Free PMC article.
-
Hydroalkylation of unactivated olefins with C(sp3)─H compounds enabled by NiH-catalyzed radical relay.Sci Adv. 2024 Dec 20;10(51):eads6885. doi: 10.1126/sciadv.ads6885. Epub 2024 Dec 18. Sci Adv. 2024. PMID: 39693419 Free PMC article.
-
Photoredox-Catalyzed Hydroalkylation of C(sp3)-H Acids.Chemistry. 2025 May 22;31(29):e202501148. doi: 10.1002/chem.202501148. Epub 2025 Apr 21. Chemistry. 2025. PMID: 40192510 Free PMC article.
References
-
- Comprehensive Organic Synthesis; Trost B. M.; Fleming I., Eds.; Pergamon, Vol. 3; 1991.
LinkOut - more resources
Full Text Sources