Hematopoietic stem cells undergo a lymphoid to myeloid switch in early stages of emergency granulopoiesis
- PMID: 37846891
- PMCID: PMC10690458
- DOI: 10.15252/embj.2023113527
Hematopoietic stem cells undergo a lymphoid to myeloid switch in early stages of emergency granulopoiesis
Abstract
Emergency granulopoiesis is the enhanced and accelerated production of granulocytes that occurs during acute infection. The contribution of hematopoietic stem cells (HSCs) to this process was reported; however, how HSCs participate in emergency granulopoiesis remains elusive. Here, using a mouse model of emergency granulopoiesis we observe transcriptional changes in HSCs as early as 4 h after lipopolysaccharide (LPS) administration. We observe that the HSC identity is changed towards a myeloid-biased HSC and show that CD201 is enriched in lymphoid-biased HSCs. While CD201 expression under steady-state conditions reveals a lymphoid bias, under emergency granulopoiesis loss of CD201 marks the lymphoid-to-myeloid transcriptional switch. Mechanistically, we determine that lymphoid-biased CD201+ HSCs act as a first response during emergency granulopoiesis due to direct sensing of LPS by TLR4 and downstream activation of NF-κΒ signaling. The myeloid-biased CD201- HSC population responds indirectly during an acute infection by sensing G-CSF, increasing STAT3 phosphorylation, and upregulating LAP/LAP* C/EBPβ isoforms. In conclusion, HSC subpopulations support early phases of emergency granulopoiesis due to their transcriptional rewiring from a lymphoid-biased to myeloid-biased population and thus establishing alternative paths to supply elevated numbers of granulocytes.
Keywords: CD201; emergency granulopoiesis; lymphoid-biased HSC; myeloid-biased HSC.
© 2023 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

- A
Graphical representation of the experimental design. WT C57BL/6 mice were injected with PBS vehicle control or LPS (35 μg) to induce emergency granulopoiesis. Four hours after injection Lin− c‐Kit+ Sca‐1+ CD48− CD150+ HSCs were sorted and subjected to scRNA‐seq.
- B
UMAP plots color‐coded for HSCs isolated from PBS‐treated (left), LPS‐treated (middle) mice and overlap (right).
- C
UMAP plot of LPS‐ and PBS‐treated transcriptomes color‐coded for the 5 phenotypes identified by graph‐based clustering.
- D
Relative contribution of PBS‐ (gray) and LPS (orange)‐treated HSCs to each cluster. X‐axis indicates percentage.
- E
Gene expression levels of top‐ranking marker genes for each cluster.
- F, G
Pseudotime trajectory of the indicated HSC phenotypes (F) and Loess regression‐smoothened gene expression of the indicated genes in pseudotime (G).
- H
Boxplot showing the enrichments of the ATAC‐seq near‐TSS peaks q‐values of top‐ranking marker genes from (E). Data shown as fold change from PBS to LPS. In the boxplot, central bands indicate median, boxes indicate1st and 3rd quartiles, and whiskers indicate furthest data point within 1.5 times the interquartile range (defined as the difference between 3rd and 1st quartile) from the appropriate quartile. Welch's t‐test independent samples with Bonferroni correction was used to assess statistical significance (**P < 0.01).
- I
Representative flow cytometry plots of multipotent progenitors in the BM cells isolated from mice treated with PBS control or LPS for 4 h, numbers indicate percentages of LKS. Dark blue boxes indicate multipotent progenitor 4 (MPP4), violet boxes indicate MPP3, green boxes indicate MPP2, and light blue boxes indicate HSCs.
- J
Quantification of panel (I). Y‐axis indicates fold change from PBS‐treated mice to LPS‐treated mice. At least five animals were included in each group. Data represent mean ± SD from two independent experiments. Two‐tailed Student's t‐test was used to assess statistical significance (**P < 0.01, ***P < 0.001, ns, not significant).

- A
UMAP plot color‐coded for the expression of Procr.
- B
Representative flow cytometry plots of BM cells isolated from mice treated with PBS control or LPS for 4 h. The x‐axes indicate CD201 expression. Numbers show percentage of CD201+ HSCs.
- C
Quantification of panel b. The y‐axis indicates percentage of CD201+ HSCs in BM.
- D
Absolute number of CD201+ HSCs in BM isolated from mice treated with PBS control or LPS for 4 h. The x‐axis indicates absolute number.
- E
Representative flow cytometry plots indicating CD201 expression in HSCs isolated from WT and Cebpb KO mice treated with PBS or LPS as indicated for 4 h. Numbers indicate percentage of CD201+ HSCs.
- F, G
Quantification of panel (E). Percentage of CD201+ HSCs (F) and CD201 mean fluorescence intensity (MFI) (G). X‐axes indicate fold change relative to PBS control mice (dashed lines).
- H
Representative flow cytometry plots indicating CD201 expression in HSCs isolated from WT and MyD88 KO mice treated with PBS or LPS as indicated for 4 h. Numbers indicate percentage of CD201+ HSCs.
- I, J
Quantification of panel (H). Percentage of CD201+ HSC (I) and CD201 MFI (J) in WT (gray columns) and MyD88 KO (pink column) mice treated with LPS for 4 h.
- K–M
Quantification of MPP4 (K), MPP3 (L) and MPP2 (M) populations in WT (gray columns) and MyD88 KO (pink column) mice treated with LPS for 4 h. X‐axes indicate the fold change to PBS control.
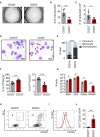
Macroscopic pictures of colony culture assays using MethoCult GF M3434 of CD201+ and CD201− HSCs isolated from WT mice. A total of 100 HSCs was plated per well. Cultures were analyzed at day 7 of culture.
Quantification of panel (A). Y‐axis indicates the absolute number of colonies per well.
Absolute cell counts per well.
Representative pictures of cells cytospun from colony culture assays. Cytospins were stained with May‐Grünwald Giemsa. Scale bar represents 20 μm. Blue arrows point at immature cells (medium to large cells with big nucleus and scant and dark‐blue cytoplasm), gray arrows at monocytes (large and round cells with round nucleus and light‐blue cytoplasm), and black arrows at granulocytes (smaller cells characterized by a ring shape or lobulated nucleus).
Differential cell counting based on cell morphology. 200–300 cells per cytospin were assessed. Y‐axis indicates the percentage of immature cells (blue bars), monocytes (gray bars), and granulocytes (black bars).
Flow cytometric analysis of cells harvested from semi‐solid cultures. Y‐axes indicate percentage of mature granulocytes (left) and immature granulocytes (right).
Quantitative RT–PCR from cells harvested from CD201+ and CD201− cultures. Expression of Mpo, Ela2, Cebpe, and Mpp9 is indicated. The y‐axis represents relative expression compared to Actb control. Each group includes values for six independent cultures.
Representative flow cytometric plots of CD201+ (left) and CD201− (right) liquid cultures. Y‐axes indicate Gr1 expression and x‐axes CD11b expression. Numbers indicate percentage of Gr1+ CD11b+ cells.
Representative histogram plot of CD201+ (gray line) and CD201− (red line) cultures. X‐axis indicates CD11b expression.
Absolute number of Gr1+ CD11b+ mature granulocytic counts in liquid cultures.

- A
Graphical representation of the experimental design. 700 CD201+ or CD201− HSCs were sorted from Ly5.2 mice and transplanted into lethally irradiated Ly5.1 recipients together with 0.5 × 106 Ly5.1 support BM.
- B
Representative flow cytometric analysis of peripheral blood from recipient mice 16 weeks after transplantation. Left panel represents a recipient mouse that received CD201+ HSCs, right panel CD201− HSCs. Y‐axes indicate percentage of Ly5.1‐derived BM support cells and y‐axes percentage of Ly5.2‐derived donor HSCs. Numbers indicate percentage of Ly5.2+ cells.
- C
Quantification of panel (B). The y‐axis indicates percentage of Ly5.2+ cells. At least five animals were included in each group.
- D
Tri‐lineage reconstitution in peripheral blood 16 weeks upon transplantation. Upper left panel indicates percentage of donor‐derived Ly5.2+ T cells, upper right panel indicates percentage of donor‐derived Ly5.2+ B cells, and lower right panel indicates percentage of donor‐derived Ly5.2+ myeloid cells. Recipient mice received CD201+ (left) or CD201− (right) HSCs.
- E
Quantification of flow cytometric data of panel (D). Y‐axis indicates the percentage of donor‐derived T cells, B cells, and myeloid cells.
- F
Abundance of lymphoid versus myeloid cells. Y‐axis indicates the lymphoid to myeloid ratio in peripheral blood derived from CD201+ or CD201− HSCs.
- G
Flow cytometric strategy to determine CD201+ and CD201− HSC‐derived progenitor and stem cells. Numbers indicate percentages of LKS. Dark blue boxes indicate multipotent progenitor 4 (MPP4), violet boxes indicate MPP3, green boxes indicate MPP2, and light blue boxes indicate HSCs.
- H–K
Quantification of panel (G). Y‐axes indicate percentages in LKS population. At least six animals were included in each group.
- L
Analysis of the BM HSC compartment isolated from mice transplanted with CD201+ (left) or CD201− (right) HSCs. Circles indicate percentage of CD201+ and CD201− HSCs upon 16 weeks of transplantation.

Schematic representation of the experimental design. Mice were treated at the indicated time points with CD201 function‐blocking antibodies (CD201 ab) and LPS (35 μg).
Quantification of MPP4, MPP3, and MPP2 populations in mice treated with LPS control (gray columns) or CD201‐function blocking antibodies followed by LPS administration (brown columns). X‐axes indicate the fold change to PBS control. Dashed lines indicate PBS levels.
Graphical representation of the experimental design. CD201+ and CD201− chimeras were challenged with LPS or PBS control 16 weeks after transplantation. Mice were sacrificed and analyzed 4 h after the challenge.
Flow cytometric analysis of BM from recipient mice that received CD201+ (top) or CD201− (bottom) HSCs and were challenged with PBS (left) or LPS (right). X‐axes indicate CD201 expression in donor‐derived (Ly5.2+) HSCs. Numbers indicate percentages.
Quantification of panel (D). Y‐axis indicates percentage of donor‐derived Ly5.2+ CD201+ HSCs in chimeras that received CD201+ or CD201− HSCs. Analysis was done 4 h after treatment. At least three animals were included in each group.
Flow cytometric analysis of BM from recipient mice that received CD201+ (top) or CD201− (bottom) HSCs and were challenge with PBS (left) or LPS (right). Flow cytometry plots indicate gating strategy to identify progenitor and stem cell populations. Dark blue boxes indicate multipotent progenitor 4 (MPP4), violet boxes indicate MPP3, green boxes indicate MPP2, and light blue boxes indicate HSCs. Numbers indicate percentages of LKS. Analysis was done 4 h after treatment.
Quantification of panel (F). Y‐axis indicates fold change from PBS‐treated mice to LPS‐treated mice. Dashed line indicates PBS levels. At least six animals were included in each group.
Graphical summary. Left panel indicates HSC behavior in resting conditions and right panel indicates HSC behavior during emergency granulopoiesis. HSCs could be divided according to CD201 expression. In steady‐state, CD201+ HSCs contribute to lymphoid production, while CD201− HSCs mainly supply granulocytic/monocytic (GM) and megakaryocytic/erythroid (Meg/E) demands. Under emergency granulopoiesis, CD201+ HSCs are transcriptionally rewired and CD201 expression is diminished, contributing to the enhanced GM and Meg/E production at the expenses of the lymphoid lineage supply.
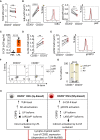
Quantification of TLR4 mean fluorescence intensity (MFI) in CD201+ (gray) and CD201− (red) HSCs.
Quantification and representative histogram plot of IκΒα (left) and p65 (right) MFI in CD201+ (gray) and CD201− (red) HSCs upon 5 min of LPS stimulation in culture (n = 6 samples per condition).
G‐CSF quantification in serum of mice treated with LPS or PBS control for 4 h. X‐axis indicates G‐CSF levels.
Quantification of G‐CSF‐receptor (G‐CSF‐R) MFI in CD201+ (gray) and CD201− (red) HSCs.
Quantification (left) and representative histogram plot (right) of pSTAT3 MFI in CD201+ (gray) and CD201− (red) HSCs upon 5 min of G‐CSF stimulation in culture (n = 6 samples per condition).
Representative flow cytometry contour plots showing levels of the distinct C/EBPβ isoforms in CD201+ and CD201− HSCs isolated from WT mice. Numbers indicate percentage of negative cells (lower left quadrant), C/EBPβ LIP isoform expressing cells (lower right quadrant), and C/EBPβ LAP/LAP* isoforms expressing cells (upper right quadrant).
Quantification of the panel (F). X‐axis indicates the percentage of the distinct C/EBPβ isoforms (n = 6 samples per condition).
Illustration summarizing the two distinct molecular mechanisms mediating emergency granulopoiesis in CD201+ and CD201− HSCs.
References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S (1998) Targeted disruption of the MyD88 gene results in loss of IL‐1‐ and IL‐18‐mediated function. Immunity 9: 143–150 - PubMed
-
- Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C et al (2012) Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell 10: 273–283 - PubMed
-
- Boettcher S, Ziegler P, Schmid MA, Takizawa H, van Rooijen N, Kopf M, Heikenwalder M, Manz MG (2012) Cutting edge: LPS‐induced emergency myelopoiesis depends on TLR4‐expressing nonhematopoietic cells. J Immunol 188: 5824–5828 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 327722/Charles University GAUK
- 101027977/EC | Horizon Europe | Excellent Science | HORIZON EUROPE Marie Sklodowska-Curie Actions (MSCA)
- 101042031/ERC Starting grant
- 5068-2022/European Molecular Biology Organization (EMBO)
- 860002/European Union Horizon 2020
- 22-18300S/Grant Agency of the Czech Republic
- RVO86652036/IBT institutional funding
- RVO 68378050/IMG institutional funding
- Japanese Society of Hematology (JSH)
- 21K08379/JSPS KAKENHI
- 21K19386/JSPS KAKENHI
- 21H02956/JSPS KAKENHI
- LX22NPO5102/National Institute for Cancer Research - EXCELES
- 2019/35/O/ST6/02484/Polish national science center
- Takeda Science Foundation (TSF)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

