Advances in gene therapy for inborn errors of immunity
- PMID: 37846903
- PMCID: PMC10621649
- DOI: 10.1097/ACI.0000000000000952
Advances in gene therapy for inborn errors of immunity
Abstract
Purpose of review: Provide an overview of the landmark accomplishments and state of the art of gene therapy for inborn errors of immunity (IEI).
Recent findings: Three decades after the first clinical application of gene therapy for IEI, there is one market authorized product available, while for several others efficacy has been demonstrated or is currently being tested in ongoing clinical trials. Gene editing approaches using programmable nucleases are being explored preclinically and could be beneficial for genes requiring tightly regulated expression, gain-of-function mutations and dominant-negative mutations.
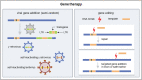
Summary: Gene therapy by modifying autologous hematopoietic stem cells (HSCs) offers an attractive alternative to allogeneic hematopoietic stem cell transplantation (HSCT), the current standard of care to treat severe IEI. This approach does not require availability of a suitable allogeneic donor and eliminates the risk of graft versus host disease (GvHD). Gene therapy can be attempted by using a viral vector to add a copy of the therapeutic gene (viral gene addition) or by using programmable nucleases (gene editing) to precisely correct mutations, disrupt a gene or introduce an entire copy of a gene at a specific locus. However, gene therapy comes with its own challenges such as safety, therapeutic effectiveness and access. For viral gene addition, a major safety concern is vector-related insertional mutagenesis, although this has been greatly reduced with the introduction of safer vectors. For gene editing, the risk of off-site mutagenesis is a main driver behind the ongoing search for modified nucleases. For both approaches, HSCs have to be manipulated ex vivo, and doing this efficiently without losing stemness remains a challenge, especially for gene editing.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Figures

References
-
- Gatti RA, Meuwissen HJ, Allen HD, et al. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet 1968; 2:1366–1369. - PubMed
-
- De Koning J, Van Bekkum DW, Dicke KA, et al. Transplantation of bone-marrow cells and fetal thymus in an infant with lymphopenic immunological deficiency. Lancet 1969; 1:1223–1227. - PubMed
-
- Hirschhorn R, Yang DR, Puck JM, et al. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nat Genet 1996; 13:290–295. - PubMed
-
- Stephan V, Wahn V, Le Deist F, et al. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N Engl J Med 1996; 335:1563–1567. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

