Ruthenium-Based Photoactivated Chemotherapy
- PMID: 37846939
- PMCID: PMC10623564
- DOI: 10.1021/jacs.3c01135
Ruthenium-Based Photoactivated Chemotherapy
Abstract
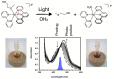
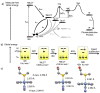
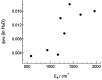
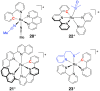
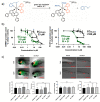
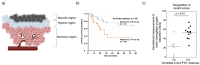
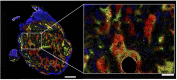
Ruthenium(II) polypyridyl complexes form a vast family of molecules characterized by their finely tuned photochemical and photophysical properties. Their ability to undergo excited-state deactivation via photosubstitution reactions makes them quite unique in inorganic photochemistry. As a consequence, they have been used, in general, for building dynamic molecular systems responsive to light but, more particularly, in the field of oncology, as prodrugs for a new cancer treatment modality called photoactivated chemotherapy (PACT). Indeed, the ability of a coordination bond to be selectively broken under visible light irradiation offers fascinating perspectives in oncology: it is possible to make poorly toxic agents in the dark that become activated toward cancer cell killing by simple visible light irradiation of the compound inside a tumor. In this Perspective, we review the most important concepts behind the PACT idea, the relationship between ruthenium compounds used for PACT and those used for a related phototherapeutic approach called photodynamic therapy (PDT), and we discuss important questions about real-life applications of PACT in the clinic. We conclude this Perspective with important challenges in the field and an outlook.
Conflict of interest statement
The author declares no competing financial interest.
Figures






References
-
- Abdel-kader M. H.The Journey of PDT Throughout History: PDT from Pharos to Present. In Comprehensive Series in Photochemical & Photobiological Sciences; Kostron H., Hasan T., Eds.; Royal Society of Chemistry: Cambridge, 2016; Chap. 1, pp 1–21. 10.1039/9781782626824-00001. - DOI
-
- Hammerle F.; Bingger I.; Pannwitz A.; Magnutzki A.; Gstir R.; Rutz A.; Wolfender J.-L.; Peintner U.; Siewert B. Targeted Isolation of Photoactive Pigments from Mushrooms Yielded a Highly Potent New Photosensitizer: 7,7′-Biphyscion. Sci. Rep. 2022, 12 (1), 1108.10.1038/s41598-022-04975-9. - DOI - PMC - PubMed
-
- Youf R.; Müller M.; Balasini A.; Thétiot F.; Müller M.; Hascoët A.; Jonas U.; Schönherr H.; Lemercier G.; Montier T.; Le Gall T. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13 (12), 1995.10.3390/pharmaceutics13121995. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

