Adeno-associated virus-vectored erythropoietin gene therapy for anemia in cats with chronic kidney disease
- PMID: 37847024
- PMCID: PMC10658539
- DOI: 10.1111/jvim.16900
Adeno-associated virus-vectored erythropoietin gene therapy for anemia in cats with chronic kidney disease
Abstract
Background: A treatment of chronic kidney disease (CKD)-associated anemia in cats is needed. SB-001 is an adeno-associated virus-vectored (AAV)-based gene therapeutic agent that is administered intramuscularly, causing the expression of feline erythropoietin.
Hypothesis/objective: We hypothesized that SB-001 injection would lead to a sustained increase in PCV in cats with CKD-associated anemia.
Animals: Twenty-three cats with International Renal Interest Society (IRIS) Stage 2 to 4 CKD-associated anemia were enrolled at 4 veterinary clinics.
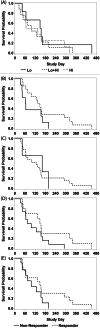
Methods: In a prospective clinical trial, cats were treated with 1 of 3 regimens of SB-001 (Lo 1.2 × 109 genome copies [GCs] on Day 0; Lo ± Hi [supplemental 2nd dose of 3.65 × 109 GC on Day 42]; Hi 3.65 × 109 GC IM on Day 0) and followed for 70 days.
Results: A response to SB-001 at any time between Day 28 and Day 70 was seen in 86% (95% confidence interval 65, 97%) of all cats. There was a significant (P < .003) increase in PCV from Day 0 to Day 28 (mean increase 6 ± 6 percentage points [pp]; n = 21), Day 42 (8 ± 9 pp; n = 21), Day 56 (10 ± 11 pp; n = 17), and Day 70 (13 ± 14 pp, n = 14). Twelve cats were hypertensive at baseline, 4 of which developed encephalopathy during the study. An additional 6 cats became hypertensive during the study.
Conclusions and clinical importance: Results of this study suggest that SB-001 therapy represents a suitable single injection treatment that can address nonregenerative anemia in cats with CKD. It was generally well tolerated; however, hypertension and encephalopathy developed in some cats as previously described in association with erythropoiesis-stimulating agent therapy.
Keywords: AAV; cats; red blood cells; renal disease.
© 2023 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Shelly Vaden serves as Associate Editor for the
Figures


Similar articles
-
Transfer of the feline erythropoietin gene to cats using a recombinant adeno-associated virus vector.Gene Ther. 2000 Mar;7(6):534-9. doi: 10.1038/sj.gt.3301126. Gene Ther. 2000. PMID: 10757028
-
A lentivirus-vectored feline erythropoietin gene therapy strategy in tissue culture and rodent models for the potential treatment of chronic renal disease-associated anemia.Am J Vet Res. 2024 Apr 20;85(6):ajvr.23.12.0280. doi: 10.2460/ajvr.23.12.0280. Print 2024 Jun 1. Am J Vet Res. 2024. PMID: 38626794
-
Expression of erythropoietin in cats treated with a recombinant adeno-associated viral vector.Am J Vet Res. 2005 Mar;66(3):450-6. doi: 10.2460/ajvr.2005.66.450. Am J Vet Res. 2005. PMID: 15822590
-
Update on Medical Management of Clinical Manifestations of Chronic Kidney Disease.Vet Clin North Am Small Anim Pract. 2016 Nov;46(6):1163-81. doi: 10.1016/j.cvsm.2016.06.004. Vet Clin North Am Small Anim Pract. 2016. PMID: 27593576 Review.
-
Therapeutics of managing reduced red cell mass associated with chronic kidney disease - Is there a case for earlier intervention?J Vet Pharmacol Ther. 2023 May;46(3):145-157. doi: 10.1111/jvp.13127. Epub 2023 Apr 10. J Vet Pharmacol Ther. 2023. PMID: 37036059 Review.
Cited by
-
Gene therapy with feline anti-Müllerian hormone analogs disrupts folliculogenesis and induces pregnancy loss in female domestic cats.Nat Commun. 2025 Feb 15;16(1):1668. doi: 10.1038/s41467-025-56924-5. Nat Commun. 2025. PMID: 39955296 Free PMC article.
References
-
- Brown CA, Elliott J, Schmiedt CW, et al. Chronic kidney disease in aged cats: clinical features, morphology, and proposed pathogeneses. Vet Pathol. 2016;53:309‐326. - PubMed
-
- Hamilton JB, Hamilton RS, Mestler GE. Duration of life and causes of death in domestic cats: influence of sex, gonadectomy, and inbreeding. J Gerontol. 1969;24:427‐437. - PubMed
-
- Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26:275‐281. - PubMed
-
- Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39:78‐85. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

