Contrast-enhanced US Evaluation of Hepatocellular Carcinoma Response to Chemoembolization: A Prospective Multicenter Trial
- PMID: 37847138
- PMCID: PMC10623205
- DOI: 10.1148/radiol.230727
Contrast-enhanced US Evaluation of Hepatocellular Carcinoma Response to Chemoembolization: A Prospective Multicenter Trial
Abstract
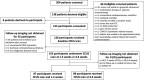
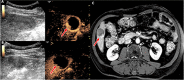
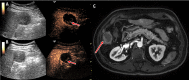
Background Contrast-enhanced (CE) US has been studied for use in the detection of residual viable hepatocellular carcinoma (HCC) after locoregional therapy, but multicenter data are lacking. Purpose To compare two-dimensional (2D) and three-dimensional (3D) CE US diagnostic performance with that of CE MRI or CT, the current clinical standard, in the detection of residual viable HCC after transarterial chemoembolization (TACE) in a prospective multicenter trial. Materials and Methods Participants aged at least 21 years with US-visible HCC scheduled for TACE were consecutively enrolled at one of three participating academic medical centers from May 2016 to March 2022. Each underwent baseline 2D and 3D CE US before TACE, 2D and 3D CE US 1-2 weeks and/or 4-6 weeks after TACE, and CE MRI or CT 4-6 weeks after TACE. CE US and CE MRI or CT were evaluated by three fellowship-trained radiologists for the presence or absence of viable tumors and were compared with reference standards of pathology (18%), angiography on re-treatment after identification of residual disease at 1-2-month follow-up imaging (31%), 4-8-month CE MRI or CT (42%), or short-term (approximately 1-2 months) CE MRI or CT if clinically decompensated and estimated viability was greater than 50% at imaging (9%). Diagnostic performance criteria, including sensitivity and specificity, were obtained for each modality and time point with generalized estimating equation analysis. Results A total of 132 participants were included (mean age, 64 years ± 7 [SD], 87 male). Sensitivity of 2D CE US 4-6 weeks after TACE was 91% (95% CI: 84, 95), which was higher than that of CE MRI or CT (68%; 95% CI: 58, 76; P < .001). Sensitivity of 3D CE US 4-6 weeks after TACE was 89% (95% CI: 81, 94), which was higher than that of CE MRI or CT (P < .001), with no evidence of a difference from 2D CE US (P = .22). CE MRI or CT had 85% (95% CI: 76, 91) specificity, higher than that of 4-6-week 2D and 3D CE US (70% [95% CI: 56, 80] and 67% [95% CI: 53, 78], respectively; P = .046 and P = .023, respectively). No evidence of differences in any diagnostic criteria were observed between 1-2-week and 4-6-week 2D CE US (P > .21). Conclusion The 2D and 3D CE US examinations 4-6 weeks after TACE revealed higher sensitivity in the detection of residual HCC than CE MRI or CT, albeit with lower specificity. Importantly, CE US performance was independent of follow-up time. Clinical trial registration no. NCT02764801 © RSNA, 2023 Supplemental material is available for this article.
Conflict of interest statement
Figures


References
-
- Sung H , Ferlay J , Siegel RL , et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries . CA Cancer J Clin 2021. ; 71 ( 3 ): 209 – 249 . - PubMed
-
- Brown DB , Nikolic B , Covey AM , et al. . Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy . J Vasc Interv Radiol 2012. ; 23 ( 3 ): 287 – 294 . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

