Profilin1 is required for prevention of mitotic catastrophe in murine and human glomerular diseases
- PMID: 37847555
- PMCID: PMC10721156
- DOI: 10.1172/JCI171237
Profilin1 is required for prevention of mitotic catastrophe in murine and human glomerular diseases
Abstract
The progression of proteinuric kidney diseases is associated with podocyte loss, but the mechanisms underlying this process remain unclear. Podocytes reenter the cell cycle to repair double-stranded DNA breaks. However, unsuccessful repair can result in podocytes crossing the G1/S checkpoint and undergoing abortive cytokinesis. In this study, we identified Pfn1 as indispensable in maintaining glomerular integrity - its tissue-specific loss in mouse podocytes resulted in severe proteinuria and kidney failure. Our results suggest that this phenotype is due to podocyte mitotic catastrophe (MC), characterized histologically and ultrastructurally by abundant multinucleated cells, irregular nuclei, and mitotic spindles. Podocyte cell cycle reentry was identified using FUCCI2aR mice, and we observed altered expression of cell-cycle associated proteins, such as p21, p53, cyclin B1, and cyclin D1. Podocyte-specific translating ribosome affinity purification and RNA-Seq revealed the downregulation of ribosomal RNA-processing 8 (Rrp8). Overexpression of Rrp8 in Pfn1-KO podocytes partially rescued the phenotype in vitro. Clinical and ultrastructural tomographic analysis of patients with diverse proteinuric kidney diseases further validated the presence of MC podocytes and reduction in podocyte PFN1 expression within kidney tissues. These results suggest that profilin1 is essential in regulating the podocyte cell cycle and its disruption leads to MC and subsequent podocyte loss.
Keywords: Cell Biology; Cell cycle; Nephrology.
Figures

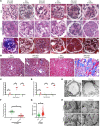
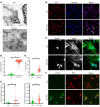


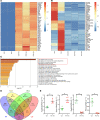


Comment in
- The loss of profilin 1 is catastrophic to podocytes doi: 10.1172/JCI175594
References
-
- Medina Rangel PX, et al. New insights into the immunity and podocyte in glomerular health and disease: from pathogenesis to therapy in proteinuric kidney disease. Integr Med Nephrol Androl. 2021;8(1):5. doi: 10.4103/imna.imna_26_21. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

