BPIFB1 loss alters airway mucus properties and diminishes mucociliary clearance
- PMID: 37847709
- PMCID: PMC11068428
- DOI: 10.1152/ajplung.00390.2022
BPIFB1 loss alters airway mucus properties and diminishes mucociliary clearance
Abstract
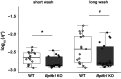
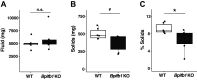
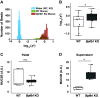
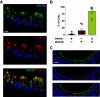
Airway mucociliary clearance (MCC) is required for host defense and is often diminished in chronic lung diseases. Effective clearance depends upon coordinated actions of the airway epithelium and a mobile mucus layer. Dysregulation of the primary secreted airway mucin proteins, MUC5B and MUC5AC, is associated with a reduction in the rate of MCC; however, how other secreted proteins impact the integrity of the mucus layer and MCC remains unclear. We previously identified the gene Bpifb1/Lplunc1 as a regulator of airway MUC5B protein levels using genetic approaches. Here, we show that BPIFB1 is required for effective MCC in vivo using Bpifb1 knockout (KO) mice. Reduced MCC in Bpifb1 KO mice occurred in the absence of defects in epithelial ion transport or reduced ciliary beat frequency. Loss of BPIFB1 in vivo and in vitro altered biophysical and biochemical properties of mucus that have been previously linked to impaired MCC. Finally, we detected colocalization of BPIFB1 and MUC5B in secretory granules in mice and the protein mesh of secreted mucus in human airway epithelia cultures. Collectively, our findings demonstrate that BPIFB1 is an important component of the mucociliary apparatus in mice and a key component of the mucus protein network.NEW & NOTEWORTHY BPIFB1, also known as LPLUNC1, was found to regulate mucociliary clearance (MCC), a key aspect of host defense in the airway. Loss of this protein was also associated with altered biophysical and biochemical properties of mucus that have been previously linked to impaired MCC.
Keywords: mucin; mucociliary clearance; mucus; rheology; viscoelasticity.
Conflict of interest statement
C. Ehre acknowledges funding from Vertex Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures



References
-
- Hill DB, Long RF, Kissner WJ, Atieh E, Garbarine IC, Markovetz MR, Fontana NC, Christy M, Habibpour M, Tarran R, Forest MG, Boucher RC, Button B. Pathological mucus and impaired mucus clearance in cystic fibrosis patients result from increased concentration, not altered pH. Eur Respir J 52: 1801297, 2018. doi:10.1183/13993003.01297-2018. - DOI - PMC - PubMed
-
- Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, Doerschuk CM, Alexis NE, Anderson WH, Henderson AG, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez F, Paine R, Woodruff PG, O'Neal WK, Boucher RC. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 377: 911–922, 2017. doi:10.1056/NEJMoa1701632. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

