Addendum to the German Consensus Recommendations on Ponatinib in the Treatment of Chronic Myeloid Leukemia
- PMID: 37848002
- PMCID: PMC11152025
- DOI: 10.1159/000533666
Addendum to the German Consensus Recommendations on Ponatinib in the Treatment of Chronic Myeloid Leukemia
Erratum in
-
Erratum.Acta Haematol. 2024;147(2):249. doi: 10.1159/000537722. Epub 2024 Feb 27. Acta Haematol. 2024. PMID: 38412838 Free PMC article. No abstract available.
Abstract
Background: Based on the new data from the primary analysis of the OPTIC (Optimizing Ponatinib Treatment in CP-CML) trial on dose optimization of ponatinib in patients with chronic phase (CP)-CML, the German consensus paper on ponatinib published in 2020 (Saussele S et al., Acta Haematol. 2020) has been updated in this addendum.
Summary: Focus is on the update of efficacy and safety of ponatinib, reflecting the new data set, as well as the update of the benefit-risk assessment and recommendations for ponatinib starting dose in CP-CML - provided that the decision to use ponatinib has already been made. Furthermore, based on OPTIC and additional empirical data, the expert panel collaborated to develop a decision tree for ponatinib dosing, specifically for intolerant and resistant patients. The recommendations on cardiovascular management have also been updated based on the most recent 2021 guidelines of the European Society of Cardiology (ESC) on cardiovascular disease prevention in clinical practice.
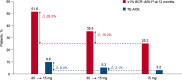
Key messages: The OPTIC data confirm the high efficacy of ponatinib in patients with CP-CML and provide the basis for individualized dose adjustment during the course of treatment.
Keywords: Cardiovascular management; Chronic myeloid leukemia; Consensus paper; Dosing regimens; Ponatinib.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Susanne Saussele has received research funding from Novartis, Bristol-Myers Squibb, and Incyte and honoraria from Bristol-Myers Squibb, Incyte, Novartis, Pfizer, and Roche. Paul La Rosée is an advisory board member for Novartis, Bristol-Myers Squibb, and Incyte and has received research funding and speaker honoraria from Novartis. Alexander Kiani has received honoraria for lectures and consulting services from Novartis, Bristol-Myers Squibb, and Pfizer. Wilhelm Haverkamp has received honoraria for lectures from Abbott, ARIAD, Amicus, ASTRA, Bayer, Daiichi Sankyo, Novartis, Pfizer, and Sanofi. Kathleen Jentsch-Ullrich has no conflicts of interest to declare. Frank Stegelmann reports honoraria from and/or consultancy for AOP Pharma, Bristol-Myers Squibb/Celgene, Incyte, Novartis, and Pfizer. Christina Rieger received speaker honoraria from and served as a consultant to AbbVie, AstraZeneca, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Incyte, Ipsen, Janssen Cilag, MSD, Novartis, and Sanofi Pasteur. Cornelius F. Waller reports honoraria for lectures from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, MSD, Novartis, Pfizer, Roche, and Takeda; support for attending meetings and/or travel from Bristol-Myers Squibb and MSD; honoraria for advisory board services from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and MSD; and consulting fees from Viatris, Roche, and Alvotech. Georg-Nikolaus Franke reports honorary/advisory board services for Incyte, Pfizer, MSD, Jazz, and Novartis and has received travel support from Gilead/Kite and Takeda. Christian Junghanss reports honoraria and research grants from Incyte, Amgen, and Novartis. Rudolf Kirchmair has received a research grant from ARIAD. Markus Theurl participated in a basic research project partially funded by ARIAD. Philipp le Coutre reports honoraria from Incyte, Novartis, and Pfizer.
Figures

References
-
- European Medicines Agency . Iclusig (ponatinib). Summary of Product characteristics; 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/iclusig-epar-....
-
- Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR::ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic Review and meta-analysis. JAMA Oncol. 2016;2(5):625–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

