Efficacy and safety of cosibelimab, an anti-PD-L1 antibody, in metastatic cutaneous squamous cell carcinoma
- PMID: 37848259
- PMCID: PMC10582968
- DOI: 10.1136/jitc-2023-007637
Efficacy and safety of cosibelimab, an anti-PD-L1 antibody, in metastatic cutaneous squamous cell carcinoma
Abstract
Background: Programmed cell death receptor-1 (PD-1)-blocking antibodies are approved to treat metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) cases ineligible for curative surgery or radiation. Notwithstanding, some patients experience inadequate responses or severe immune-related adverse events (AEs), indicating the need for improved therapies. Cosibelimab is a high-affinity programmed cell death-ligand 1 (PD-L1)-blocking antibody that activates innate and adaptive immunity by blocking PD-L1 interaction with PD-1 and B7-1 receptors. It is an unmodified immunoglobulin G1 subtype with a functional Fc domain capable of inducing antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Here, we present results of the pivotal study of patients with metastatic CSCC from an open-label, multicenter, multiregional, multicohort, phase 1 trial of cosibelimab.
Methods: In this trial, participants with metastatic CSCC received cosibelimab 800 mg intravenously every 2 weeks. Primary endpoint was objective response rate (ORR) by independent central review using Response Evaluation Criteria in Solid Tumors, V.1.1. Secondary endpoints included duration of response (DOR) and safety.
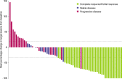
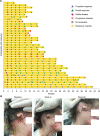
Results: Objective response was observed in 37 of 78 participants (47.4% (95% CI: 36.0% to 59.1%)), with median follow-up of 15.4 months (range: 0.4 to 40.5) as of data cut-off. Median DOR was not reached (range: 1.4+ to 34.1+ months), with response ongoing in 73.0% of participants. Common treatment-emergent AEs (≥15%) were fatigue (26.9%), rash (16.7%), and anemia (15.4%). Eighteen participants (23.1%) experienced immune-related AEs (grade 3: n=2 (2.6%); no grade 4/5). No treatment-related deaths were reported.
Conclusions: Cosibelimab demonstrated clinically meaningful ORR and DOR and was associated with a manageable safety profile.
Trial registration number: NCT03212404.
Keywords: Immune Checkpoint Inhibitors; Immunotherapy; Programmed Cell Death 1 Receptor; Skin Neoplasms.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PC has nothing to disclose. RL has received honoraria from AstraZeneca/MedImmune, Bristol Myers Squibb Foundation, MSD, and Sanofi; has served as a consultant and/or scientific advisor for AstraZeneca/MedImmune and Roche; and has received compensation for travel, accommodations, and other expenses from MSD. DB has nothing to disclose. DLH has served as a consultant and/or scientific advisor for Checkpoint Therapeutics. MM has nothing to disclose. SA has nothing to disclose. JC has nothing to disclose. SF has nothing to disclose. AK has nothing to disclose. DRM has nothing to disclose. AM has nothing to disclose. VSh has served as a speaker for Elekta. HS has nothing to disclose. AT has nothing to disclose. M-AB-G has served as a consultant and/or scientific advisor for BMS, Eisai, MSD, Novartis, and Pierre Fabre and has received research funding from Novartis. CC has received grant/research support from AstraZeneca, Novartis, and Roche. SD has received institutional research grants from BMS and MSD; has served as a consultant and/or scientific advisor for BMS, MSD, and Pierre Fabre; and has received compensation for congress attendance from BMS and MSD. AD has nothing to disclose. OD has nothing to disclose. PK has nothing to disclose. IL has financial or non-financial interests in Agenus, BMS, Janssen, MacroGenics, MSD, Pfizer, and Roche. HM has received honoraria from BMS, Merck Serono, MSD, Novartis, and Pierre Fabre; has received institutional support from BMS, Canceropole PACA, and Société Française de Dermatologie; has served as a consultant for BMS, Merck Serono, MSD, Novartis, and Pierre Fabre; has served on a data safety monitoring board/advisory board for Novartis and Pierre Fabre; and has received travel grants from Novartis and Pierre Fabre. EM-C has served as a consultant and/or scientific advisor for MBS, MSD, Novartis, Pierre Fabre, and Sanofi; has received honoraria from MBS, MSD, Novartis, Pierre Fabre, and Sanofi; and has received compensation for travel, accommodations, and other expenses from MSD. VSr has nothing to disclose. JO is an employee of Checkpoint Therapeutics and holds stocks and other ownership interests in the company; has served in leadership roles at Checkpoint Therapeutics; and has received compensation for travel, accommodations, and other expenses from Checkpoint Therapeutics. JD has served as a consultant and/or scientific advisor for Amgen, Bayer, BeiGene, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Merck KGaA, Novartis, Novotech, Pfizer, Pierre Fabre, and Roche/Genentech; has served on a data safety monitoring board/advisory board for Boehringer Ingelheim and Pfizer; has served as a board director for Cancer Trials Australia and ANZSA; and has received institutional grant/research support from AstraZeneca/MedImmune, BeiGene, BMS, GlaxoSmithKline, Lilly, Novartis, and Roche.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
