Lysosomes in retinal health and disease
- PMID: 37848361
- PMCID: PMC10842632
- DOI: 10.1016/j.tins.2023.09.006
Lysosomes in retinal health and disease
Abstract
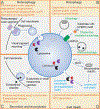
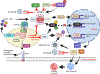
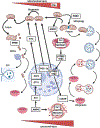
Lysosomes play crucial roles in various cellular processes - including endocytosis, phagocytosis, and autophagy - which are vital for maintaining retinal health. Moreover, these organelles serve as environmental sensors and act as central hubs for multiple signaling pathways. Through communication with other cellular components, such as mitochondria, lysosomes orchestrate the cytoprotective response essential for preserving cellular homeostasis. This coordination is particularly critical in the retina, given its high metabolic rate and susceptibility to photo-oxidative stress. Consequently, impaired lysosomal function and dysregulated communication between lysosomes and other organelles contribute significantly to the pathobiology of major retinal degenerative diseases. This review explores the pivotal role of lysosomes in retinal cells and their involvement in retinal degenerative diseases.
Keywords: age-related macular degeneration; glaucoma; lysosome membrane permeabilization; lysosome–mitochondria crosstalk; mTOR signaling; retinitis pigmentosa.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests D.S. has patents on Cryba1 and TFEB as therapies for eye-related diseases. The other authors declare no competing interests.
Figures

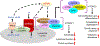
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

