Endogenous renal adiponectin drives gluconeogenesis through enhancing pyruvate and fatty acid utilization
- PMID: 37848446
- PMCID: PMC10582045
- DOI: 10.1038/s41467-023-42188-4
Endogenous renal adiponectin drives gluconeogenesis through enhancing pyruvate and fatty acid utilization
Abstract
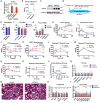
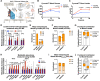
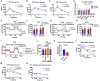
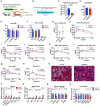
Adiponectin is a secretory protein, primarily produced in adipocytes. However, low but detectable expression of adiponectin can be observed in cell types beyond adipocytes, particularly in kidney tubular cells, but its local renal role is unknown. We assessed the impact of renal adiponectin by utilizing male inducible kidney tubular cell-specific adiponectin overexpression or knockout mice. Kidney-specific adiponectin overexpression induces a doubling of phosphoenolpyruvate carboxylase expression and enhanced pyruvate-mediated glucose production, tricarboxylic acid cycle intermediates and an upregulation of fatty acid oxidation (FAO). Inhibition of FAO reduces the adiponectin-induced enhancement of glucose production, highlighting the role of FAO in the induction of renal gluconeogenesis. In contrast, mice lacking adiponectin in the kidney exhibit enhanced glucose tolerance, lower utilization and greater accumulation of lipid species. Hence, renal adiponectin is an inducer of gluconeogenesis by driving enhanced local FAO and further underlines the important systemic contribution of renal gluconeogenesis.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Croset M, et al. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50:740–746. - PubMed
-
- Mather, A. & Pollock, C. Glucose handling by the kidney. Kidney Int Suppl, S1-S6. 10.1038/ki.2010.509, (2011). - PubMed
-
- Mithieux G, et al. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am. J. Physiol. Endocrinol. Metab. 2004;286:E370–E375. - PubMed
-
- Meyer C, et al. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am. J. Physiol. Endocrinol. Metab. 2002;282:E428–E434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 DK133630/DK/NIDDK NIH HHS/United States
- R01 DK078184/DK/NIDDK NIH HHS/United States
- P30 DK127984/DK/NIDDK NIH HHS/United States
- R03 DK135783/DK/NIDDK NIH HHS/United States
- R01 DK115703/DK/NIDDK NIH HHS/United States
- R01 DK099110/DK/NIDDK NIH HHS/United States
- R01 DK131537/DK/NIDDK NIH HHS/United States
- R01 DK055758/DK/NIDDK NIH HHS/United States
- R01 DK127274/DK/NIDDK NIH HHS/United States
- RC2 DK118620/DK/NIDDK NIH HHS/United States
- K01 DK125447/DK/NIDDK NIH HHS/United States
- R01 DK081423/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

