Indicators to evaluate quality of care in head and neck cancer in Spain
- PMID: 37848694
- PMCID: PMC11026290
- DOI: 10.1007/s12094-023-03298-z
Indicators to evaluate quality of care in head and neck cancer in Spain
Abstract
Purpose: This study aimed to develop a set of criteria and indicators to evaluate the quality of care of patients with head and neck cancer (HNC).
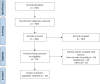
Methods: A systematic literature review was conducted to identify valuable criteria/indicators for the assessment of the quality of care in HNC. With the aid of a technical group, a scientific committee of oncologists specialised in HNC used selected criteria to propose indicators that were evaluated with a two-round Delphi method. Indicators on which consensus was achieved were then prioritised by the scientific committee to develop a final set of indicators.
Results: We proposed a list of 50 indicators used in the literature or developed by us to be evaluated with a Delphi method. There was consensus on the appropriateness of 47 indicators in the first round; the remaining 3 achieved consensus in the second round. The 50 indicators were scored to prioritise them, leading to a final selection of 29 indicators related to structure (3), process (22), or outcome (4) and covering diagnosis, treatment, follow-up, and health outcomes in patients with HNC. Easy-to-use index cards were developed for each indicator, with their criterion, definition, formula for use in real-world clinical practice, rationale, and acceptable level of attainment.
Conclusions: We have developed a set of 29 evidence-based and expert-supported indicators for evaluating the quality of care in HNC, covering diagnosis, treatment, follow-up, and health outcomes.
Keywords: Criteria; Delphi; Head and neck cancer; Indicators; Quality of care; Spain.
© 2023. The Author(s).
Conflict of interest statement
Virginia Arrazubi Arrula declares receiving honoraria for participation in advisory boards from MSD and BMS and Merck; and travel, accommodations and expenses from MSD, Merck and Lilly. Yolanda Escobar Álvarez reports receiving honoraria for participation in advisory boards from MSD and BMS and Merck; and travel, accommodations and expenses from MSD, Merck and BMS. Almudena García Castaño reports receiving honoraria for participation in advisory boards from MSD, BMS and Novartis; and travel, accommodations and expenses from MSD, BMS, Merk and Pierre Fabre. Lara Iglesias Docampo has received advisory honoraria from Merck, MSD, BMS, Lilly, and Roche; speaker fees from Merck, MSD, BMS, Roche, Bayer, and Sanofi; and travel, accommodations and expenses from Merck and MSD. Julio Lambea Sorrosal has received honoraria for participation in advisory boards from MSD, BMS and Merck; and travel, accommodations and expenses from MSD, BMS, and Merck. Pedro Pérez Segura has received honoraria for participation in advisory boards from MSD, BMS and Merck; and travel, accommodations and expenses from MSD, BMS, and Merck. Antonio Rueda Domínguez has received honoraria for participation in advisory boards from MSD, BMS and Merck; and travel, accommodations and expenses from MSD, BMS, and Merck. Rafael López has received honoraria for participation in advisory boards from Roche, AstraZeneca, Merck, MSD, Bayer, BMS, Novartis, Janssen, Lilly, Pfizer and Leo; travel, accommodations and expenses from Pharmamar, Roche, BMS and Pierre Fabre; research funding from Roche and Merck; and is the co-founder and a shareholder in Nasasbiotech, Diversa Technologies. Juan Jesús Cruz Hernández, Juan José Grau de Castro, Francisco J. Campos-Lucas, Irene Santamaría Rodríguez, Maria Bessa, Paula Gratal, Fernando Caballero-Martínez, Diana Monge Martín, and Cristina Antón-Rodríguez have no competing interests to declare that are relevant to the content of this article.
Figures


References
-
- Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2020;31:1462–1475. doi: 10.1016/j.annonc.2020.07.011. - DOI - PubMed
-
- National Comprehensive Cancer Network. NCCN Guidelines: Head and neck cancer v2.2022. 2022
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

