Vascular Grafts: Technology Success/Technology Failure
- PMID: 37849668
- PMCID: PMC10521696
- DOI: 10.34133/bmef.0003
Vascular Grafts: Technology Success/Technology Failure
Abstract
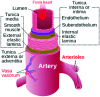
Vascular prostheses (grafts) are widely used for hemodialysis blood access, trauma repair, aneurism repair, and cardiovascular reconstruction. However, smaller-diameter (≤4 mm) grafts that would be valuable for many reconstructions have not been achieved to date, although hundreds of papers on small-diameter vascular grafts have been published. This perspective article presents a hypothesis that may open new research avenues for the development of small-diameter vascular grafts. A historical review of the vascular graft literature and specific types of vascular grafts is presented focusing on observations important to the hypothesis to be presented. Considerations in critically reviewing the vascular graft literature are discussed. The hypothesis that perhaps the "biocompatible biomaterials" comprising our vascular grafts-biomaterials that generate dense, nonvascularized collagenous capsules upon implantation-may not be all that biocompatible is presented. Examples of materials that heal with tissue reconstruction and vascularity, in contrast to the fibrotic encapsulation, are offered. Such prohealing materials may lead the way to a new generation of vascular grafts suitable for small-diameter reconstructions.
Copyright © 2023 Buddy Ratner.
Conflict of interest statement
Competing interests: The author is a founder and stockholder in Healionics Corporation.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

