Exacerbations and Real-World Outcomes After Single-Inhaler Triple Therapy of Budesonide/Glycopyrrolate/Formoterol Fumarate, Among Patients with COPD: Results from the EROS (US) Study
- PMID: 37849918
- PMCID: PMC10577086
- DOI: 10.2147/COPD.S432963
Exacerbations and Real-World Outcomes After Single-Inhaler Triple Therapy of Budesonide/Glycopyrrolate/Formoterol Fumarate, Among Patients with COPD: Results from the EROS (US) Study
Erratum in
-
Erratum: Exacerbations and Real-World Outcomes After Single-Inhaler Triple Therapy of Budesonide/Glycopyrrolate/Formoterol Fumarate, Among Patients with COPD: Results from the EROS (US) Study [Corrigendum].Int J Chron Obstruct Pulmon Dis. 2024 Aug 6;19:1799-1800. doi: 10.2147/COPD.S484253. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39129963 Free PMC article.
Abstract
Purpose: Triple therapy to prevent exacerbations from chronic obstructive pulmonary disease (COPD) is associated with improved health compared to single and dual-agent therapy in some populations. This study assessed the benefits of prompt administration of budesonide/glycopyrrolate/formoterol fumarate (BGF) following a COPD exacerbation.
Patients and methods: EROS was a retrospective analysis of people with COPD using the MORE2 Registry®. Inclusion required ≥1 severe, ≥2 moderate, or ≥1 moderate exacerbation while on other maintenance treatment. Within 12 months following the index exacerbation, ≥1 pharmacy claim for BGF was required. Primary outcomes were the rate of COPD exacerbations and healthcare costs for those that received BGF promptly (within 30 days of index exacerbation) versus delayed (31-180 days) and very delayed (181-365 days). The effect of each 30-day delay in initiation of BGF was estimated using a multivariable negative binomial regression model.
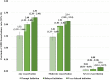
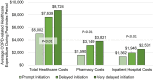
Results: 2409 patients were identified: 434 prompt, 1187 delayed, and 788 very delayed. The rate (95% CI) of total exacerbations post-index increased as time to BGF initiation increased: prompt 1.52 (1.39-1.66); delayed 2.00 (1.92-2.09); and very delayed 2.30 (2.20-2.40). Adjusting for patient characteristics, each 30-day delay in receiving BGF was associated with a 5% increase in the average number of subsequent exacerbations (rate ratio, 95% CI: 1.05, 1.01-1.08; p<0.05). Prompt initiation of BGF was also associated with lower post-index annualized COPD-related costs ($5002 for prompt vs $7639 and $8724 for the delayed and very delayed groups, respectively).
Conclusion: Following a COPD exacerbation, promptly initiating BGF was associated with a reduction in subsequent exacerbations and reduced healthcare utilization and costs.
Keywords: COPD; budesonide/glycopyrrolate/formoterol fumarate; delayed therapy; exacerbations; triple therapy.
© 2023 Strange et al.
Conflict of interest statement
SS, SP, DE, NF, and MP are employees of AstraZeneca and hold AstraZeneca stock. JT, JS, BL, and BA are employees of Inovalon, who received funding from AstraZeneca to conduct this study. DRT, EP, and CS are paid consultants of AstraZeneca. DRT reports personal fees from Stage Analytics, eMax Health, Horizon Pharmaceuticals, Monument Analytics; grants from Takeda Pharmaceuticals, outside the submitted work. CS reports personal fees from AlphaNet, Adverum, CSL Behring, Morair, UpToDate, GlaxoSmithKline; grants from Arrowhead, OCI, Pandorum, Pulmanage, Takeda, Vertex, NuVaira, CSA Medical, Grifols, Pulmonx, and AstraZeneca, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures




References
-
- Mannino RM, Mapel D, Zhang Q, et al. Publications list. JMCP. 2023;29:1.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

