A photoactivatable and phenylboronic acid-functionalized nanoassembly for combating multidrug-resistant gram-negative bacteria and their biofilms
- PMID: 37849944
- PMCID: PMC10578387
- DOI: 10.1093/burnst/tkad041
A photoactivatable and phenylboronic acid-functionalized nanoassembly for combating multidrug-resistant gram-negative bacteria and their biofilms
Abstract
Background: Multidrug-resistant (MDR) gram-negative bacteria-related infectious diseases have caused an increase in the public health burden and mortality. Moreover, the formation of biofilms makes these bacteria difficult to control. Therefore, developing novel interventions to combat MDR gram-negative bacteria and their biofilms-related infections are urgently needed. The purpose of this study was to develop a multifunctional nanoassembly (IRNB) based on IR-780 and N, N'-di-sec-butyl-N, N'- dinitroso-1,4-phenylenediamine (BNN6) for synergistic effect on the infected wounds and subcutaneous abscesses caused by gram-negative bacteria.
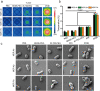
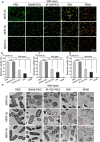
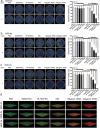
Methods: The characterization and bacteria-targeting ability of IRNB were investigated. The bactericidal efficacy of IRNB against gram-negative bacteria and their biofilms was demonstrated by crystal violet staining assay, plate counting method and live/dead staining in vitro. The antibacterial efficiency of IRNB was examined on a subcutaneous abscess and cutaneous infected wound model in vivo. A cell counting kit-8 assay, Calcein/PI cytotoxicity assay, hemolysis assay and intravenous injection assay were performed to detect the biocompatibility of IRNB in vitro and in vivo.
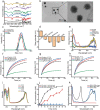
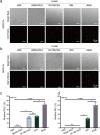
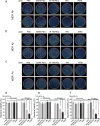
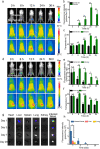
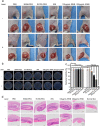
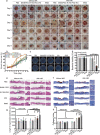
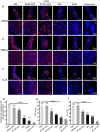
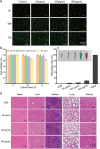
Results: Herein, we successfully developed a multifunctional nanoassembly IRNB based on IR-780 and BNN6 for synergistic photothermal therapy (PTT), photodynamic therapy (PDT) and nitric oxide (NO) effect triggered by an 808 nm laser. This nanoassembly could accumulate specifically at the infected sites of MDR gram-negative bacteria and their biofilms via the covalent coupling effect. Upon irradiation with an 808 nm laser, IRNB was activated and produced both reactive oxygen species (ROS) and hyperthermia. The local hyperthermia could induce NO generation, which further reacted with ROS to generate ONOO-, leading to the enhancement of bactericidal efficacy. Furthermore, NO and ONOO- could disrupt the cell membrane, which converts bacteria to an extremely susceptible state and further enhances the photothermal effect. In this study, IRNB showed a superior photothermal-photodynamic-chemo (NO) synergistic therapeutic effect on the infected wounds and subcutaneous abscesses caused by gram-negative bacteria. This resulted in effective control of associated infections, relief of inflammation, promotion of re-epithelization and collagen deposition, and regulation of angiogenesis during wound healing. Moreover, IRNB exhibited excellent biocompatibility, both in vitro and in vivo.
Conclusions: The present research suggests that IRNB can be considered a promising alternative for treating infections caused by MDR gram-negative bacteria and their biofilms.
Keywords: Boronic acid; Multidrug-resistant gram-negative bacteria; Nitric oxide; Photodynamic therapy; Photothermal therapy; Synergistic.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures

References
-
- Centers for Disease Control and Prevention (U.S.) . Antibiotic resistance threats in the United States. 2019;2019. 10.15620/cdc:82532. - DOI
-
- Falcone M, Paterson D. Spotlight on ceftazidime/avibactam: a new option for MDR gram-negative infections. J Antimicrob Chemoth. 2016;71:2713–22. - PubMed
-
- Cook MA, Wright GD. The past, present, and future of antibiotics. Sci Transl Med. 2022;14:eabo7793. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

