The leptin receptor has no role in delta-cell control of beta-cell function in the mouse
- PMID: 37850099
- PMCID: PMC10577419
- DOI: 10.3389/fendo.2023.1257671
The leptin receptor has no role in delta-cell control of beta-cell function in the mouse
Abstract
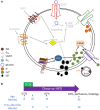
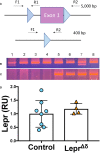
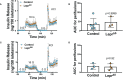
Introduction: Leptin inhibits insulin secretion from isolated islets from multiple species, but the cell type that mediates this process remains elusive. Several mouse models have been used to explore this question. Ablation of the leptin receptor (Lepr) throughout the pancreatic epithelium results in altered glucose homeostasis and ex vivo insulin secretion and Ca2+ dynamics. However, Lepr removal from neither alpha nor beta cells mimics this result. Moreover, scRNAseq data has revealed an enrichment of LEPR in human islet delta cells.
Methods: We confirmed LEPR upregulation in human delta cells by performing RNAseq on fixed, sorted beta and delta cells. We then used a mouse model to test whether delta cells mediate the diminished glucose-stimulated insulin secretion in response to leptin.
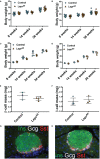
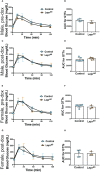
Results: Ablation of Lepr within mouse delta cells did not change glucose homeostasis or insulin secretion, whether mice were fed a chow or high-fat diet. We further show, using a publicly available scRNAseq dataset, that islet cells expressing Lepr lie within endothelial cell clusters.
Conclusions: In mice, leptin does not influence beta-cell function through delta cells.
Keywords: beta cells; beta-cell activity; delta cells; differential expression (DE); leptin receptor (LEPR); mouse models.
Copyright © 2023 Zhang, Katada, Mosleh, Yuhas, Peng and Golson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

