Octane in exhaled breath to diagnose acute respiratory distress syndrome in invasively ventilated intensive care unit patients
- PMID: 37850212
- PMCID: PMC10577595
- DOI: 10.1183/23120541.00214-2023
Octane in exhaled breath to diagnose acute respiratory distress syndrome in invasively ventilated intensive care unit patients
Abstract
Background: The concentration of exhaled octane has been postulated as a reliable biomarker for acute respiratory distress syndrome (ARDS) using metabolomics analysis with gas chromatography and mass spectrometry (GC-MS). A point-of-care (POC) breath test was developed in recent years to accurately measure octane at the bedside. The aim of the present study was to validate the diagnostic accuracy of exhaled octane for ARDS using a POC breath test in invasively ventilated intensive care unit (ICU) patients.
Methods: This was an observational cohort study of consecutive patients receiving invasive ventilation for at least 24 h, recruited in two university ICUs. GC-MS and POC breath tests were used to quantify the exhaled octane concentration. ARDS was assessed by three experts following the Berlin definition and used as the reference standard. The area under the receiver operating characteristic curve (AUC) was used to assess diagnostic accuracy.
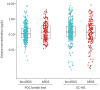
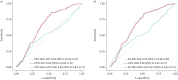
Results: 519 patients were included and 190 (37%) fulfilled the criteria for ARDS. The median (interquartile range) concentration of octane using the POC breath test was not significantly different between patients with ARDS (0.14 (0.05-0.37) ppb) and without ARDS (0.11 (0.06-0.26) ppb; p=0.64). The AUC for ARDS based on the octane concentration in exhaled breath using the POC breath test was 0.52 (95% CI 0.46-0.57). Analysis of exhaled octane with GC-MS showed similar results.
Conclusions: Octane in exhaled breath has insufficient diagnostic accuracy for ARDS. This disqualifies the use of octane as a biomarker in the diagnosis of ARDS and challenges most of the research performed up to now in the field of exhaled breath metabolomics.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: L.D.J. Bos reports grants from the Dutch Lung Foundation (Young Investigator grant and Dirkje Postma Award), the Dutch Lung Foundation and Health Holland (public–private partnership grant), and the IMI COVID19 initiative, and an Amsterdam UMC fellowship, a ZonMW COVID-19 Urgency grant and the ERS Gold Metal for ARDS; he reports participating in advisory boards for Sobi, Exvastat, Santhera, Pfizer and AstraZeneca, all paid to his institution, and consultancy for Scailyte, Santhera and Janssen & Janssen, all paid to his institution, outside the submitted work. A.R.M. Verschueren, T.M.E. Nijsen, I. Geven, C.N. Presură and R. Rietman are employees of Philips Research. L.A. Hagens, N.F.L. Heijnen, M.R. Smit, D.W. Fenn, P. Brinkman, M.J. Schultz, D.C.J.J. Bergmans and R.M. Schnabel have no conflicts of interest to declare.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous
