Cellular Heterogeneity of Activated Primary Human Macrophages and Associated Drug-Gene Networks: From Biology to Precision Therapeutics
- PMID: 37850387
- PMCID: PMC10624416
- DOI: 10.1161/CIRCULATIONAHA.123.064794
Cellular Heterogeneity of Activated Primary Human Macrophages and Associated Drug-Gene Networks: From Biology to Precision Therapeutics
Abstract
Background: Interferon-γ (IFNγ) signaling plays a complex role in atherogenesis. IFNγ stimulation of macrophages permits in vitro exploration of proinflammatory mechanisms and the development of novel immune therapies. We hypothesized that the study of macrophage subpopulations could lead to anti-inflammatory interventions.
Methods: Primary human macrophages activated by IFNγ (M(IFNγ)) underwent analyses by single-cell RNA sequencing, time-course cell-cluster proteomics, metabolite consumption, immunoassays, and functional tests (phagocytic, efferocytotic, and chemotactic). RNA-sequencing data were analyzed in LINCS (Library of Integrated Network-Based Cellular Signatures) to identify compounds targeting M(IFNγ) subpopulations. The effect of compound BI-2536 was tested in human macrophages in vitro and in a murine model of atherosclerosis.
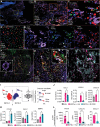
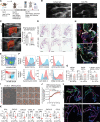
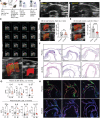
Results: Single-cell RNA sequencing identified 2 major clusters in M(IFNγ): inflammatory (M(IFNγ)i) and phagocytic (M(IFNγ)p). M(IFNγ)i had elevated expression of inflammatory chemokines and higher amino acid consumption compared with M(IFNγ)p. M(IFNγ)p were more phagocytotic and chemotactic with higher Krebs cycle activity and less glycolysis than M(IFNγ)i. Human carotid atherosclerotic plaques contained 2 such macrophage clusters. Bioinformatic LINCS analysis using our RNA-sequencing data identified BI-2536 as a potential compound to decrease the M(IFNγ)i subpopulation. BI-2536 in vitro decreased inflammatory chemokine expression and secretion in M(IFNγ) by shrinking the M(IFNγ)i subpopulation while expanding the M(IFNγ)p subpopulation. BI-2536 in vivo shifted the phenotype of macrophages, modulated inflammation, and decreased atherosclerosis and calcification.
Conclusions: We characterized 2 clusters of macrophages in atherosclerosis and combined our cellular data with a cell-signature drug library to identify a novel compound that targets a subset of macrophages in atherosclerosis. Our approach is a precision medicine strategy to identify new drugs that target atherosclerosis and other inflammatory diseases.
Keywords: atherosclerosis; inflammation; macrophages; multiomics; phagocytosis; systems biology.
Conflict of interest statement
Figures





References
-
- Decano JL, Singh SA, Gasparotto Bueno C, Ho Lee L, Halu A, Chelvanambi S, Matamalas JT, Zhang H, Mlynarchik AK, Qiao J, et al. . Systems approach to discovery of therapeutic targets for vein graft disease: PPARα pivotally regulates metabolism, activation, and heterogeneity of macrophages and lesion development. Circulation. 2021;143:2454–2470. doi: 10.1161/circulationaha.119.043724 - PMC - PubMed
-
- Nakano T, Katsuki S, Chen M, Decano JL, Halu A, Lee LH, Pestana DVS, Kum AST, Kuromoto RK, Golden WS, et al. . Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-Notch signaling. Circulation. 2019;139:78–96. doi: 10.1161/circulationaha.118.034588 - PMC - PubMed
-
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/nejmoa1707914 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

