An archaeal virus-encoded anti-CRISPR protein inhibits type III-B immunity by inhibiting Cas RNP complex turnover
- PMID: 37850639
- PMCID: PMC10681719
- DOI: 10.1093/nar/gkad804
An archaeal virus-encoded anti-CRISPR protein inhibits type III-B immunity by inhibiting Cas RNP complex turnover
Erratum in
-
Correction to 'An archaeal virus-encoded anti-CRISPR protein inhibits type III-B immunity by inhibiting Cas RNP complex turnover'.Nucleic Acids Res. 2023 Dec 11;51(22):12537. doi: 10.1093/nar/gkad1107. Nucleic Acids Res. 2023. PMID: 37953382 Free PMC article. No abstract available.
Abstract
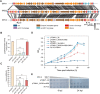
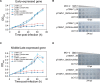
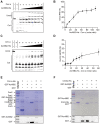
CRISPR-Cas systems are widespread in prokaryotes and provide adaptive immune against viral infection. Viruses encode a type of proteins called anti-CRISPR to evade the immunity. Here, we identify an archaeal virus-encoded anti-CRISPR protein, AcrIIIB2, that inhibits Type III-B immunity. We find that AcrIIIB2 inhibits Type III-B CRISPR-Cas immunity in vivo regardless of viral early or middle-/late-expressed genes to be targeted. We also demonstrate that AcrIIIB2 interacts with Cmr4α subunit, forming a complex with target RNA and Cmr-α ribonucleoprotein complex (RNP). Furtherly, we discover that AcrIIIB2 inhibits the RNase activity, ssDNase activity and cOA synthesis activity of Cmr-α RNP in vitro under a higher target RNA-to-Cmr-α RNP ratio and has no effect on Cmr-α activities at the target RNA-to-Cmr-α RNP ratio of 1. Our results suggest that once the target RNA is cleaved by Cmr-α RNP, AcrIIIB2 probably inhibits the disassociation of cleaved target RNA, therefore blocking the access of other target RNA substrates. Together, our findings highlight the multiple functions of a novel anti-CRISPR protein on inhibition of the most complicated CRISPR-Cas system targeting the genes involved in the whole life cycle of viruses.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Molecular basis for inhibition of type III-B CRISPR-Cas by an archaeal viral anti-CRISPR protein.Cell Host Microbe. 2023 Nov 8;31(11):1837-1849.e5. doi: 10.1016/j.chom.2023.10.003. Epub 2023 Oct 30. Cell Host Microbe. 2023. PMID: 37909049
-
Inhibition of Type III CRISPR-Cas Immunity by an Archaeal Virus-Encoded Anti-CRISPR Protein.Cell. 2019 Oct 3;179(2):448-458.e11. doi: 10.1016/j.cell.2019.09.003. Epub 2019 Sep 26. Cell. 2019. PMID: 31564454
-
A seed motif for target RNA capture enables efficient immune defence by a type III-B CRISPR-Cas system.RNA Biol. 2019 Sep;16(9):1166-1178. doi: 10.1080/15476286.2019.1618693. Epub 2019 May 26. RNA Biol. 2019. PMID: 31096876 Free PMC article.
-
Anti-CRISPR Proteins in Archaea.Trends Microbiol. 2020 Nov;28(11):913-921. doi: 10.1016/j.tim.2020.05.007. Epub 2020 Jun 1. Trends Microbiol. 2020. PMID: 32499102 Review.
-
CRISPR-Cas adaptive immune systems in Sulfolobales: genetic studies and molecular mechanisms.Sci China Life Sci. 2021 May;64(5):678-696. doi: 10.1007/s11427-020-1745-0. Epub 2020 Oct 29. Sci China Life Sci. 2021. PMID: 33140167 Review.
Cited by
-
Characterization of Five CRISPR Systems in Microcystis aeruginosa FACHB-524 with Focus on the In Vitro Antiviral Activity of One CRISPR System.Int J Mol Sci. 2025 Feb 12;26(4):1554. doi: 10.3390/ijms26041554. Int J Mol Sci. 2025. PMID: 40004028 Free PMC article.
-
The Cas10 nuclease activity relieves host dormancy to facilitate spacer acquisition and retention during type III-A CRISPR immunity.bioRxiv [Preprint]. 2024 Feb 12:2024.02.11.579731. doi: 10.1101/2024.02.11.579731. bioRxiv. 2024. Update in: Cell Host Microbe. 2024 Dec 11;32(12):2050-2062.e6. doi: 10.1016/j.chom.2024.11.005. PMID: 38405743 Free PMC article. Updated. Preprint.
-
Natively expressed AcrIII-1 does not function as an anti-CRISPR protein.Nature. 2025 Apr;640(8059):E8-E14. doi: 10.1038/s41586-025-08649-0. Epub 2025 Apr 16. Nature. 2025. PMID: 40240860 No abstract available.
-
Cas10 relieves host growth arrest to facilitate spacer retention during type III-A CRISPR-Cas immunity.Cell Host Microbe. 2024 Dec 11;32(12):2050-2062.e6. doi: 10.1016/j.chom.2024.11.005. Epub 2024 Dec 2. Cell Host Microbe. 2024. PMID: 39626678 Free PMC article.
-
AcrIIIA1 is a protein-RNA anti-CRISPR complex that targets core Cas and accessory nucleases.Nucleic Acids Res. 2024 Dec 11;52(22):13490-13514. doi: 10.1093/nar/gkae1006. Nucleic Acids Res. 2024. PMID: 39551936 Free PMC article.
References
-
- Wang J.Y., Pausch P., Doudna J.A.. Structural biology of CRISPR-Cas immunity and genome editing enzymes. Nat. Rev. Microbiol. 2022; 20:641–656. - PubMed
-
- Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D.A., Horvath P.. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007; 315:1709–1712. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

