Mitochondrial metabolism in Drosophila macrophage-like cells regulates body growth via modulation of cytokine and insulin signaling
- PMID: 37850733
- PMCID: PMC10695174
- DOI: 10.1242/bio.059968
Mitochondrial metabolism in Drosophila macrophage-like cells regulates body growth via modulation of cytokine and insulin signaling
Abstract
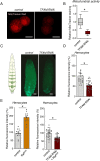
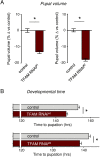
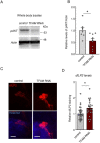
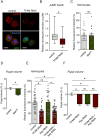
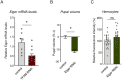
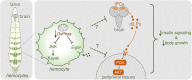
Macrophages play critical roles in regulating and maintaining tissue and whole-body metabolism in normal and disease states. While the cell-cell signaling pathways that underlie these functions are becoming clear, less is known about how alterations in macrophage metabolism influence their roles as regulators of systemic physiology. Here, we investigate this by examining Drosophila macrophage-like cells called hemocytes. We used knockdown of TFAM, a mitochondrial genome transcription factor, to reduce mitochondrial OxPhos activity specifically in larval hemocytes. We find that this reduction in hemocyte OxPhos leads to a decrease in larval growth and body size. These effects are associated with a suppression of systemic insulin, the main endocrine stimulator of body growth. We also find that TFAM knockdown leads to decreased hemocyte JNK signaling and decreased expression of the TNF alpha homolog, Eiger in hemocytes. Furthermore, we show that genetic knockdown of hemocyte JNK signaling or Eiger expression mimics the effects of TFAM knockdown and leads to a non-autonomous suppression of body size without altering hemocyte numbers. Our data suggest that modulation of hemocyte mitochondrial metabolism can determine their non-autonomous effects on organismal growth by altering cytokine and systemic insulin signaling. Given that nutrient availability can control mitochondrial metabolism, our findings may explain how macrophages function as nutrient-responsive regulators of tissue and whole-body physiology and homeostasis.
Keywords: Drosophila; Cytokine TNF-α/Eiger; Hemocytes; Insulin signaling; JNK signaling; Metabolism; Mitochondria; OxPhos; Systemic growth; TFAM.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
References
-
- Andersen, D. S., Colombani, J., Palmerini, V., Chakrabandhu, K., Boone, E., Rothlisberger, M., Toggweiler, J., Basler, K., Mapelli, M., Hueber, A. O.et al. (2015). The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature 522, 482-486. 10.1038/nature14298 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

