JNK regulates ciliogenesis through the interflagellar transport complex and actin networks
- PMID: 37851005
- PMCID: PMC10585068
- DOI: 10.1083/jcb.202303052
JNK regulates ciliogenesis through the interflagellar transport complex and actin networks
Abstract
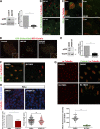
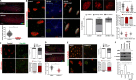
The c-Jun N-terminal kinase (JNK) regulates various important physiological processes. Although the JNK pathway has been under intense investigation for over 20 yr, its complexity is still perplexing, with multiple protein partners underlying the diversity of its activity. We show that JNK is associated with the basal bodies in both primary and motile cilia. Loss of JNK disrupts basal body migration and docking and leads to severe ciliogenesis defects. JNK's involvement in ciliogenesis stems from a dual role in the regulation of the actin networks of multiciliated cells (MCCs) and the establishment of the intraflagellar transport-B core complex. JNK signaling is also critical for the maintenance of the actin networks and ciliary function in mature MCCs. JNK is implicated in the development of diabetes, neurodegeneration, and liver disease, all of which have been linked to ciliary dysfunction. Our work uncovers a novel role of JNK in ciliogenesis and ciliary function that could have important implications for JNK's role in the disease.
© 2023 Chatzifrangkeskou et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








References
-
- Björkblom, B., Padzik A., Mohammad H., Westerlund N., Komulainen E., Hollos P., Parviainen L., Papageorgiou A.C., Iljin K., Kallioniemi O., et al. 2012. c-Jun N-terminal kinase phosphorylation of MARCKSL1 determines actin stability and migration in neurons and in cancer cells. Mol. Cell. Biol. 32:3513–3526. 10.1128/MCB.00713-12 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

