Telemedicine Buprenorphine Initiation and Retention in Opioid Use Disorder Treatment for Medicaid Enrollees
- PMID: 37851446
- PMCID: PMC10585416
- DOI: 10.1001/jamanetworkopen.2023.36914
Telemedicine Buprenorphine Initiation and Retention in Opioid Use Disorder Treatment for Medicaid Enrollees
Abstract
Importance: Early COVID-19 mitigation strategies placed an additional burden on individuals seeking care for opioid use disorder (OUD). Telemedicine provided a way to initiate and maintain transmucosal buprenorphine treatment of OUD.
Objective: To examine associations between transmucosal buprenorphine OUD treatment modality (telemedicine vs traditional) during the COVID-19 public health emergency and the health outcomes of treatment retention and opioid-related nonfatal overdose.
Design, setting, and participants: This retrospective cohort study was conducted using Medicaid claims and enrollment data from November 1, 2019, to December 31, 2020, for individuals aged 18 to 64 years from Kentucky and Ohio. Data were collected and analyzed in June 2022, with data updated during revision in August 2023.
Exposures: The primary exposure of interest was the modality of the transmucosal buprenorphine OUD treatment initiation. Relevant patient demographic and comorbidity characteristics were included in regression models.
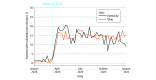
Main outcomes and measures: There were 2 main outcomes of interest: retention in treatment after initiation and opioid-related nonfatal overdose after initiation. For outcomes measured after initiation, a 90-day follow-up period was used. The main analysis used a new-user study design; transmucosal buprenorphine OUD treatment initiation was defined as initiation after more than a 60-day gap in buprenorphine treatment. In addition, uptake of telemedicine for buprenorphine was examined, overall and within patients initiating treatment, across quarters in 2020.
Results: This study included 41 266 individuals in Kentucky (21 269 women [51.5%]; mean [SD] age, 37.9 [9.0] years) and 50 648 individuals in Ohio (26 425 women [52.2%]; mean [SD] age, 37.1 [9.3] years) who received buprenorphine in 2020, with 18 250 and 24 741 people initiating buprenorphine in Kentucky and Ohio, respectively. Telemedicine buprenorphine initiations increased sharply at the beginning of 2020. Compared with nontelemedicine initiation, telemedicine initiation was associated with better odds of 90-day retention with buprenorphine in both states (Kentucky: adjusted odds ratio, 1.13 [95% CI, 1.01-1.27]; Ohio: adjusted odds ratio, 1.19 [95% CI, 1.06-1.32]) in a regression analysis adjusting for patient demographic and comorbidity characteristics. Telemedicine initiation was not associated with opioid-related nonfatal overdose (Kentucky: adjusted odds ratio, 0.89 [95% CI, 0.56-1.40]; Ohio: adjusted odds ratio, 1.08 [95% CI, 0.83-1.41]).
Conclusions and relevance: In this cohort study of Medicaid enrollees receiving buprenorphine for OUD, telemedicine buprenorphine initiation was associated with retention in treatment early during the COVID-19 pandemic. These findings add to the literature demonstrating positive outcomes associated with the use of telemedicine for treatment of OUD.
Trial registration: ClinicalTrials.gov NCT04111939.
Conflict of interest statement
Figures



Comment in
-
Navigating the Path to Effective, Equitable, and Evidence-Based Telehealth for Opioid Use Disorder Treatment.JAMA Netw Open. 2023 Oct 2;6(10):e2336885. doi: 10.1001/jamanetworkopen.2023.36885. JAMA Netw Open. 2023. PMID: 37851449 No abstract available.
References
-
- Ohio Department of Health . Director’s stay at home order. Published online March 22, 2020. Accessed August 15, 2023. https://content.govdelivery.com/attachments/OHOOD/2020/03/22/file_attach...
-
- Ohio Department of Health . Governor DeWine announces details of Ohio’s Responsible RestartOhio Plan. Published online April 27, 2020. Accessed August 15, 2023. https://coronavirus.ohio.gov/resources/news-releases-news-you-can-use/go...
-
- Kentucky Governor Andy Beshear . Kentucky’s response to COVID-19. Published online October 20, 2020. Accessed August 15, 2023. https://governor.ky.gov/Documents/20201020_COVID-19_page-archive.pdf
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

