Janus Kinase Inhibitors and Adverse Events of Acne: A Systematic Review and Meta-Analysis
- PMID: 37851459
- PMCID: PMC10585588
- DOI: 10.1001/jamadermatol.2023.3830
Janus Kinase Inhibitors and Adverse Events of Acne: A Systematic Review and Meta-Analysis
Abstract
Importance: Janus kinase (JAK) inhibitors are increasingly used across a range of dermatologic conditions. Adverse events of acne have been noted in some studies in clinical practice, but the scope of this outcome across JAK inhibitors has not been established.
Objective: To systematically analyze all published phase 2 and 3 placebo-controlled randomized clinical trials (RCTs) of JAK inhibitors for the risk of acne as an adverse effect of these medications.
Data sources: Comprehensive search of Ovid MEDLINE and PubMed databases through January 31, 2023.
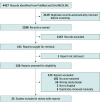
Study selection: Inclusion criteria were phase 2 and 3 placebo-controlled RCTs of JAK inhibitors published in English with reported adverse events of acne.
Data extraction and synthesis: Two reviewers independently reviewed and extracted information from all included studies.
Main outcomes and measures: The primary outcome of interest was the incidence of acne following JAK inhibitor use. A meta-analysis was conducted using random-effects models.
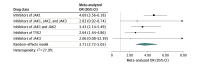
Results: A total of 25 unique studies (10 839 unique participants; 54% male and 46% female) were included in the final analysis. The pooled odds ratio (OR) was calculated to be 3.83 (95% CI, 2.76-5.32) with increased ORs for abrocitinib (13.47 [95% CI, 3.25-55.91]), baricitinib (4.96 [95% CI, 2.52-9.78]), upadacitinib (4.79 [95% CI, 3.61-6.37]), deucravacitinib (2.64 [95% CI, 1.44-4.86]), and deuruxolitinib (3.30 [95% CI, 1.22-8.93]). Estimated ORs were higher across studies investigating the use of JAK inhibitors for the management of dermatologic compared with nondermatologic conditions (4.67 [95% CI, 3.10-7.05]) as well as for JAK1-specific inhibitors (4.69 [95% CI, 3.56-6.18]), combined JAK1 and JAK2 inhibitors (3.43 [95% CI, 2.14-5.49]), and tyrosine kinase 2 inhibitors (2.64 [95% CI, 1.44-4.86]).
Conclusions and relevance: In this systematic review and meta-analysis, JAK inhibitor use was associated with an elevated odds of acne. Patients should be properly counseled on this potential adverse effect of these medications before treatment initiation. Future studies are needed to further elucidate the pathophysiology of this association.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

