Unraveling the promoter effect and the roles of counterion exchange in glycosylation reaction
- PMID: 37851803
- PMCID: PMC10584349
- DOI: 10.1126/sciadv.adk0531
Unraveling the promoter effect and the roles of counterion exchange in glycosylation reaction
Abstract
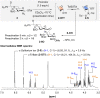
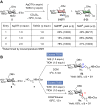
The stereoselectivity of glycosidic bond formation continues to pose a noteworthy hurdle in synthesizing carbohydrates, primarily due to the simultaneous occurrence of SN1 and SN2 processes during the glycosylation reaction. Here, we applied an in-depth analysis of the glycosylation mechanism by using low-temperature nuclear magnetic resonance and statistical approaches. A pathway driven by counterion exchanges and reaction byproducts was first discovered to outline the stereocontributions of intermediates. Moreover, the relative reactivity values, acceptor nucleophilic constants, and Hammett substituent constants (σ values) provided a general index to indicate the mechanistic pathways. These results could allow building block tailoring and reaction condition optimization in carbohydrate synthesis to be greatly facilitated and simplified.
Figures






References
-
- Y. Maki, R. Okamoto, M. Izumi, Y. Kajihara, Chemical synthesis of an erythropoietin glycoform having a triantennary N-glycan: Significant change of biological activity of glycoprotein by addition of a small molecular weight trisaccharide. J. Am. Chem. Soc. 142, 20671–20679 (2020). - PubMed
LinkOut - more resources
Full Text Sources

