Plasma TNFRSF11B as a New Predictive Inflammatory Marker of Sepsis-ARDS with Endothelial Dysfunction
- PMID: 37851947
- PMCID: PMC10629264
- DOI: 10.1021/acs.jproteome.3c00576
Plasma TNFRSF11B as a New Predictive Inflammatory Marker of Sepsis-ARDS with Endothelial Dysfunction
Abstract
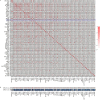
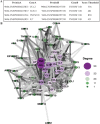
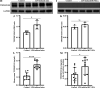
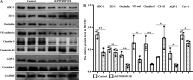
Inflammation plays an important role in the development of sepsis-acute respiratory distress syndrome (ARDS). Olink inflammation-related biomarker panels were used to analyze the levels of 92 inflammation-related proteins in plasma with sepsis-ARDS (n = 25) and healthy subjects (n = 25). There were significant differences in 64 inflammatory factors, including TNFRSF11B in sepsis-ARDS, which was significantly higher than that in controls. Functional analysis showed that TNFRSF11B was closely focused on signal transduction, immune response, and inflammatory response. The TNFRSF11B level in sepsis-ARDS plasma, LPS-induced mice, and LPS-stimulated HUVECs significantly increased. The highest plasma concentration of TNFRSF11B in patients with sepsis-ARDS was 10-20 ng/mL, and 10 ng/mL was selected to stimulate HUVECs. Western blot results demonstrated that the levels of syndecan-1, claudin-5, VE-cadherin, occludin, aquaporin-1, and caveolin-1 in TNFRSF11B-stimulated HUVECs decreased, whereas that of connexin-43 increased in TNFRSF11B-stimulated HUVECs. To the best of the authors' knowledge, this study was the first to reveal elevated TNFRSF11B in sepsis-ARDS associated with vascular endothelial dysfunction. In summary, TNFRSF11B may be a new potential predictive and diagnostic biomarker for vascular endothelium damage in sepsis-ARDS.
Keywords: TNFRSF11B; endothelial dysfunction; glycocalyx; junctions; sepsis−ARDS.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome.Crit Care. 2013 Oct 24;17(5):R253. doi: 10.1186/cc13080. Crit Care. 2013. PMID: 24156650 Free PMC article.
-
Crocin alleviates lipopolysaccharide-induced acute respiratory distress syndrome by protecting against glycocalyx damage and suppressing inflammatory signaling pathways.Inflamm Res. 2020 Mar;69(3):267-278. doi: 10.1007/s00011-019-01314-z. Epub 2020 Jan 10. Inflamm Res. 2020. PMID: 31925528 Free PMC article.
-
Vascular endothelial cadherin expression in lung specimens of patients with sepsis-induced acute respiratory distress syndrome and endothelial cell cultures.Pathobiology. 2013;80(5):245-51. doi: 10.1159/000347062. Epub 2013 Apr 27. Pathobiology. 2013. PMID: 23635392
-
Serum HMGB1 and soluble urokinase plasminogen activator receptor levels aid diagnosis and prognosis prediction of sepsis with acute respiratory distress syndrome.Biomark Med. 2023 Feb;17(4):231-239. doi: 10.2217/bmm-2022-0899. Epub 2023 May 9. Biomark Med. 2023. PMID: 37158106 Review.
-
From sepsis to acute respiratory distress syndrome (ARDS): emerging preventive strategies based on molecular and genetic researches.Biosci Rep. 2020 May 29;40(5):BSR20200830. doi: 10.1042/BSR20200830. Biosci Rep. 2020. PMID: 32319516 Free PMC article. Review.
Cited by
-
Identification of a multi-omics factor predictive of long COVID in the IMPACC study.bioRxiv [Preprint]. 2025 Feb 14:2025.02.12.637926. doi: 10.1101/2025.02.12.637926. bioRxiv. 2025. PMID: 39990442 Free PMC article. Preprint.
-
The glycocalyx: a key target for treatment of severe acute pancreatitis-associated multiple organ dysfunction syndrome.Hum Cell. 2025 May 24;38(4):107. doi: 10.1007/s13577-025-01227-6. Hum Cell. 2025. PMID: 40411704 Free PMC article. Review.
-
Systematic Review of Interleukin-35 in Endothelial Dysfunction: A New Target for Therapeutic Intervention.Mediators Inflamm. 2025 Feb 12;2025:2003124. doi: 10.1155/mi/2003124. eCollection 2025. Mediators Inflamm. 2025. PMID: 39974277 Free PMC article.
-
TNFSF11/TNFRSF11A Axis Amplifies HDM-Induced Airway Remodeling by Strengthening TGFβ1/STAT3 Action.Allergy Asthma Immunol Res. 2024 Jul;16(4):399-421. doi: 10.4168/aair.2024.16.4.399. Allergy Asthma Immunol Res. 2024. PMID: 39155739 Free PMC article.
-
Baseline predictors for 28-day COVID-19 severity and mortality among hospitalized patients: results from the IMPACC study.Front Med (Lausanne). 2025 Jul 4;12:1604388. doi: 10.3389/fmed.2025.1604388. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40687705 Free PMC article.
References
-
- LaRivière W. B.; Schmidt E. P.. The pulmonary endothelial glycocalyx in ARDS: a critical role for heparan sulfate. In Current Topics in Membranes; Elsevier, 2018; Vol. 82, pp 33–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

